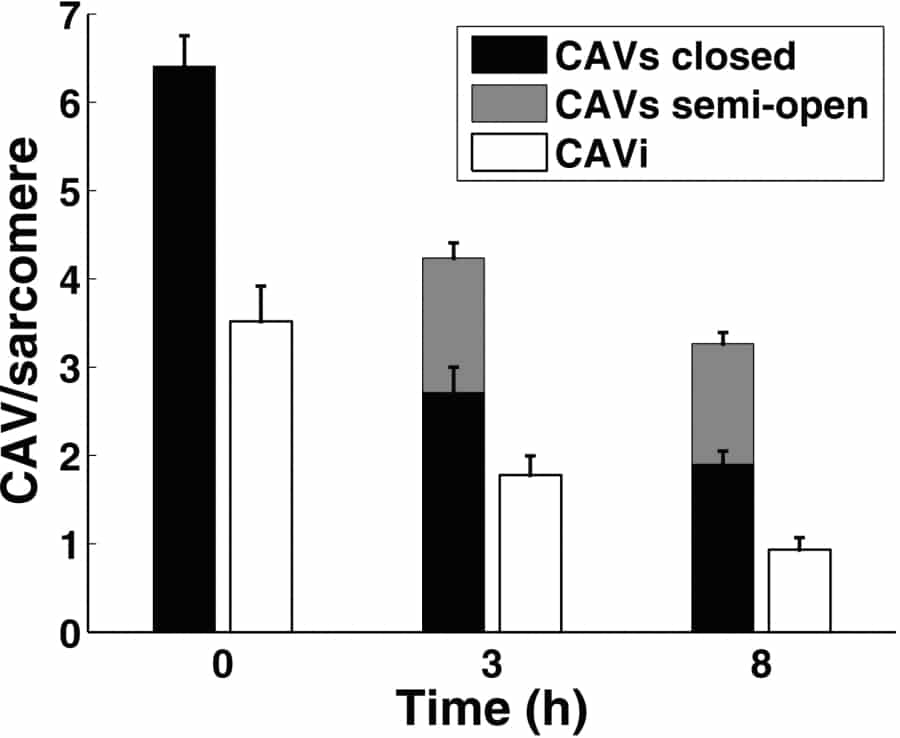
Introduction: Caveolae (CAV) are centres of cardiac signal transduction, often studied in isolated cardiomyocytes (M). Little is known about CAV preservation in isolated M, so we monitored this for up to 8h after cell isolation. Methods: Left ventricular M were isolated from New Zealand (NZ) white rabbit (<1kg) after Schedule 1 killing (conforming to UK Home Office regulations). After excision, the heart was swiftly connected to a Langendorff system, perfused with physiological saline, then cardioplegically arrested (high K+), followed by enzymatic digestion (L-type collagenase; Sigma-Aldrich). Isolated M were stored in saline with BSA and trypsin inhibitor at 22°C (pH 7.4) until fixation with Karnovsky’s formaldehyde-glutaraldehyde mix at 0h, 3h or 8h after isolation. M were resin embedded, sectioned longitudinally (80nm), and imaged by transmission electron microscopy. CAV in direct contact or ‘within reach’ of the sarcolemma (CAVs; centre <50nm from sarcolemma) were distinguished from internal ones (CAVi). For each M, CAVs and CAVi were quantified within a 1μm sub-sarcolemmal space of 9-12 sarcomeres (6 M at each time point). Kruskal-Wallis and Ranksum tests were used (p<0.05). Results: The density of CAVi and CAVs decreases with time after isolation (Fig.1). The proportion of CAVs increases from 66% at 0h (70% at 3h) to 80% at 8h. At 0h, no CAVs were in a ‘semi-open state’, i.e. with wide connection between CAV lumen and extracellular space, while this sub-group increased at later time points (36% of CAVs at 3h, 42% of CAVs at 8h). Discussion: Isolated M experience CAV loss. This may be caused by either (or both) internalization of CAVs and degradation of CAVi, or progressive sarcolemmal incorporation of CAVs replenished by CAVi. Sarcolemmal integration of CAVs (‘semi-open fraction’) has been seen during stretch and swelling of Langendorff-perfused rabbit hearts (Kohl et al. 2003). This, and more recent observations on stretch-induced redistribution of CAV proteins into the sarcolemma (Calaghan & White 2008), suggests that mechanical factors contribute to CAV destabilization. Cell isolation and storage may provide such mechanical clues (cell swelling in saline). Membrane-integration of CAV can have potentially relevant effects, from increased sarcolemmal surface area and loss of CAV ‘lumen’, to exposure of proteins to novel environments and destruction of functional microdomains. Conclusion: Significant remodelling of CAV occurs in M within a time relevant for single cell research.
University of Cambridge (2008) Proc Physiol Soc 11, PC17
Poster Communications: Caveolar remodelling in rabbit left ventricular myocytes after cell isolation
R. A. Burton1, G. K. Picton1, M. Fink1, P. Camelliti1, T. Mansoori2, C. Bollensdorff1, J. Sheldon3, G. Bub1, P. Kohl1
1. Physiology, Anatomy, Genetics, University of Oxford, Oxford, Oxfordshire, United Kingdom. 2. Computing Laboratory, University of Oxford, Oxford, Oxfordshire, United Kingdom. 3. Biological & Molecular Sciences, Oxford Brookes University, Oxford, Oxfordshire, United Kingdom.
View other abstracts by:
Fig. 1: Decline in CAV numbers per sarcomere (in 1μm sub-sarcolemmal space) with time after cell isolation.
Where applicable, experiments conform with Society ethical requirements.