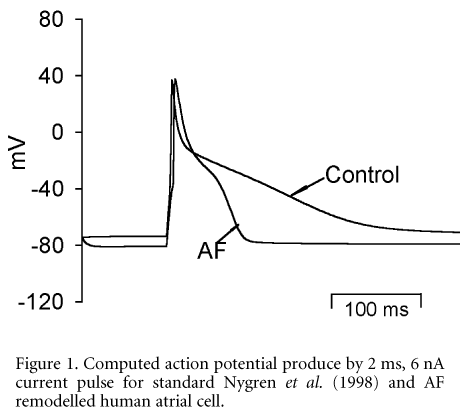
The functional mechanisms underlying chronic atrial fibrillation (AF) have been described in animal models (Nattel et al. 2000) and in humans (Bosch et al. 1999). AF is associated with remodelling of ionic channel densities and kinetics, and gap junctional coupling. Remodelling of ionic channels has been proposed to underlie the marked decrease in the action potential duration (APD) and adaptation of the APD restitution curve as well as a decrease in atrial conduction velocity. These are believed to favour the genesis and maintenance of chronic AF by multiple re-entrant wavelets. We used a computational model to test the hypothesis that remodelling of ionic channel properties is quantitatively sufficient to account for the reported changes in APD and APD restitution curves of human atrial cells in chronic AF. The model of Nygren et al. (1998) of electrical activity of human atrial cells was modified to incorporate the experimental data of AF-induced changes in ionic channel conductances for iK,1 (increased by 250 %), iCa,L (decreased by 74 %), ito (decreased by 85 %) and kinetics of ito (activation curve shifted by 16 mV), iCa,L (time constant of fast inactivation increased by 62 %) and iNa (inactivation curve shifted by 10 mV). Action potentials and APD restitution curves for remodelled cells were computed and compared with those obtained for the standard model, and also compared quantitatively with those obtained experimentally. In the model we found that AF induced changes in the ionic channel conductances and kinetics were able to quantitatively reproduce the changes in the APD and APD restitution curve seen in clinical experiments. The AF induced changes in the characteristics of human atrial action potential, such as shortening APD, increase of resting potential (towards more negative) and adaptation of APD restitution curve are quantitatively accounted for by the integrated actions of AF up-regulation of iK,1, downregulation of ito, iCa,L and changed channel kinetics of iCa,L, ito and iNa.