Background: Gravity-dependent shifts in central blood volume (CBV) induced by the microgravity of orbital spaceflight pose unique physiological challenges for the astronaut brain. Recent attention has focused on gravity-induced redistribution of fluids toward the head and associated haemostatic consequences associated with altered regional cerebral perfusion (Marshall-Goebel et al., 2019). Changes in posture in terrestrial analogues (stand to head-down tilt) allows for the opportunity to induce a large gravity-dependent shift in CBV to better phenotype underlying mechanisms. Furthermore, the future of human space exploration will require extended extravehicular activities and consequent exposure to low levels of oxygen (hypoxia) that has been associated with blood brain-barrier disruption subject to regional cerebral hyperperfusion and activated coagulation (Bailey et al., 2009; Bailey et al., 2020). The present study aimed to determine to what extent cephalad fluid shifts independently, or in conjunction with inspiratory hypoxia, collectively impact clot microstructure and potential links to altered regional cerebral perfusion.
Methods: Ten healthy males aged 30 (mean) ± 9 (SD) years old were recruited into a randomised, single-blind, counterbalanced study involving two separate trials separated by 60 min washout. They were examined in two different postural positions (standing head-up and 180° head-down tilt) for 10 min each in normoxia (FIO2 = 20.93 %) and hypoxia (FIO2 = 12 %). Changes in CBV via thoracic impedance were measured using a tetrapolar high-resolution impedance monitor (THRIM 2994D, UFI, Morro Bay, CA, USA), according to established methods (Bailey et al., 2020). Anterior (internal carotid artery, ICAQ) and posterior (vertebral artery, VAQ) blood flow was assessed using duplex ultrasound. Global cerebral blood flow (gCBF) was calculated as (ICAQ + VAQ) × 2. Shear rate (SR) was calculated as 4 × peak envelope velocity/ arterial diameter. Cerebrovascular conductance index (CVCi) was calculated as Q/mean arterial blood pressure. Cephalic venous blood was obtained without stasis for haemorheological assessment of the fractal dimension (df), a novel biomarker of insipient clot microstructure. Following confirmation of distribution normality (Shapiro W Wilks tests), data were analysed using a 2-way (Trial × Position) repeated measures ANOVA and Bonferonni-corrected paired samples t-tests.
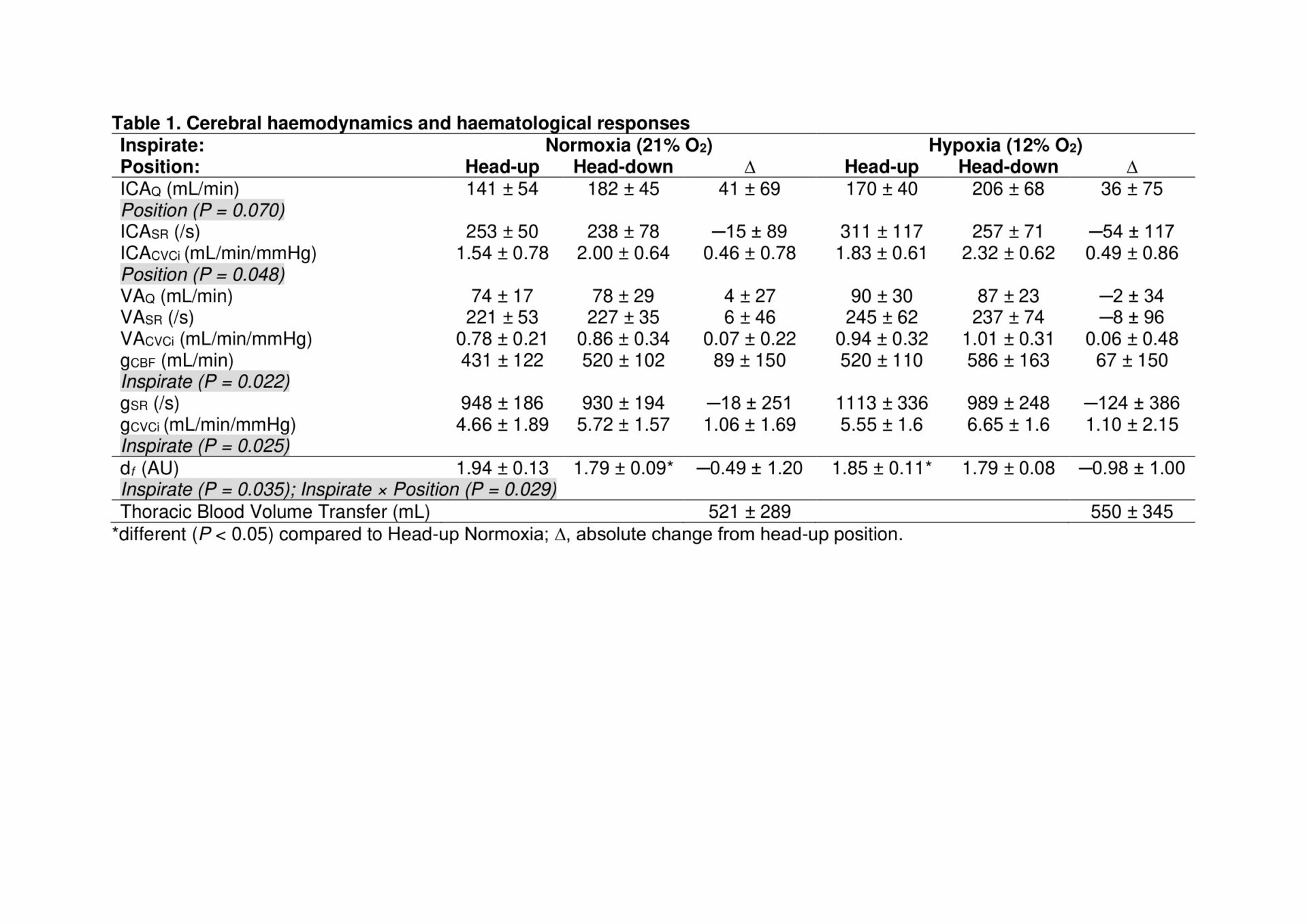
Results: Head-down tilt was generally associated with an increase in thoracic blood volume (p=<0.001) and consequent elevation in ICACVCi (p=0.048) due primarily to an increase in ICAQ that was not apparent in the posterior circulation (unchanged VAQ/CVCi, Table 1). Thoracic blood volume transfer between head-up and head-down tilt was not compounded by hypoxia (p=0.870). Despite global hypoxic cerebral vasodilation (elevated gCBF, p=0.022), this did not affect the regional responses to postural tilt. Hypoxia increased df (p=0.035) with a general and consistent reduction observed during head-down tilt.
Conclusions: These findings collectively demonstrate constrained perfusion to the posterior cerebral circulation and consistent reduction in activated coagulation, reflected by a reduction in incipient clot viscoelastic strength, polymerisation and crosslinking. These changes were independent of systemic oxygenation status and may collectively confer neuroprotective benefits against hyperperfusion-induced structural-functional damage to the neurovascular unit.