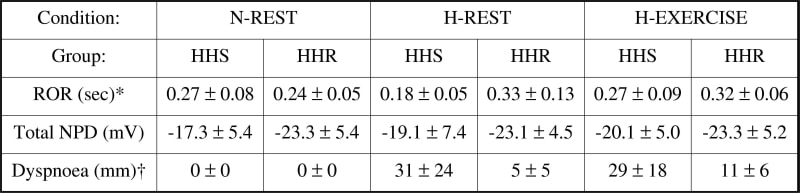
A reduction in cerebral oxygenation may contribute to brain swelling and neurovascular headache (Bailey et al. 2005) which in severe cases may progress to pulmonary oedema. This suggests that remote pulmonary complications may have a neurogenic basis. The present study combined exercise and hypoxia to effect changes in cerebral oxygen (O2) delivery and examined implications for cerebral autoregulation and pulmonary function in individuals susceptible to hypoxic headache (HHS) compared to those who are resistant (HHR). Eighteen males aged 26 ± 6 years (mean ± SD) were examined at rest in normoxia (N-REST), after 6h passive exposure to 12% O2 (H-REST) followed by a maximal cycling test (H-EXERCISE). Middle cerebral artery blood flow velocity (transcranial doppler) and mean arterial blood pressure (plethysmography) were recorded continuously during an acute hypotensive challenge to calculate a dynamic rate of cerebral autoregulation (ROR). Transepithelial nasal potential difference (NPD) recordings were obtained as an indirect measure of alveolar ion transport. Dyspnoea and headache ratings were recorded according to established clinical guidelines. Six subjects were excluded from overall analyses due to experimental complications. Table 1 identifies that cerebral autoregulation was impaired in the HHS group as indicated by the greater decrease in ROR during passive exposure to hypoxia and lack of increase following exercise. Dyspnoea ratings were elevated and a negative relationship was observed between the increase (H-REST minus N-REST) in ratings and decrease in ROR (r = -0.81, P = 0.05). In contrast, there were no detectable differences observed in NPD. These findings suggest that a transient impairment in cerebral autoregulation may contribute to the sensation of breathlessness in hypoxia and exercise. The lack of change in NPD tentatively excludes interstitial pulmonary oedema as a contributory factor.
University College London December 2005 (2006) Proc Physiol Soc 1, PC27
Poster Communications: Cerebral haemodynamics during exercise and hypoxia; ‘downstream’ consequences for pulmonary function
Evans, Kevin A; Ainslie, Philip N; Fall, Lewis; Martins, Pedro; Kewley, Emily; Mason, Nicholas P; Bailey, Damian M;
1. Department of Physiology, University of Glamorgan, Pontypridd, United Kingdom. 2. Department of Physiology, University of Otago, Dunedin, New Zealand. 3. Department of Critical Care Medicine, University Hospital of Wales, Cardiff, United Kingdom.
View other abstracts by:
Table 1. Cerebro-pulmonary function during hypoxia and exercise in HHSValues are means ± SD; HHS (n = 6); HHR (n = 6); *main effect for group and main effects for condition and group (P < 0.05 two-way repeated measures ANOVA)
Where applicable, experiments conform with Society ethical requirements.