Neuroblastoma cells undergo differentiation in response to retinoids (Redfern et al. 1995) and this is the basis of retinoid-based chemotherapy regimes currently in clinical trials for treatment of Neuroblastoma disease. However, not all cells respond to treatment and little is known about the mechanisms underlying this heterogeneity. Since changes in the concentration of intracellular calcium ions ([Ca2+]i) have been hypothesised to be involved in the differentiation of neuroblastoma cells (Celli et al. 1999), we are investigating whether changes in Ca2+ signalling mechanisms occur following differentiation of SH-SY5Y cells with 9-cis retinoic acid (9cRA).
In order to investigate changes in putative Ca2+ entry mechanisms we have used fura-2 fluorescence measurements to monitor both changes in [Ca2+]i and divalent cation entry (Mn2+ quench). Fluorescence from fura-2 AM-loaded cells was continuously monitored using a Perkin-Elmer LS-50B fluorimeter with excitation and emission wavelengths of 340 and 510 nm, respectively, for monitoring changes in [Ca2+]i, and 360 and 510 nm for Mn2+ quench (Bennett et al. 1998).
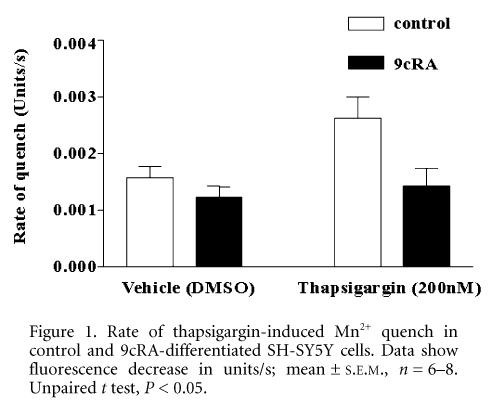
In the presence of extracellular Ca2+ the muscarinic agonist methacholine stimulated a rise in [Ca2+]i in both control and differentiated cells. The EC50 values were similar, 6.8 and 7.1 mM methacholine in control and differentiated cells respectively. However, in the absence of extracellular Ca2+, the EC50 value in differentiated cells was less than half that in control cells (11.6 and 4.7 mM; control and differentiated cells, respectively). In order to investigate whether this effect may reflect a down-regulation of a Ca2+ entry pathway, we used the Mn2+ quench technique to directly monitor divalent cation entry induced by the Ca2+-ATPase inhibitor thapsigargin, a classical activator of capacitative Ca2+ entry (CCE).
The results (Fig. 1) showed that both control and differentiated cells exhibit basal levels of Mn2+ entry that are not significantly different (P = 0.24). However, upon thapsigargin stimulation, control cells showed a significant increase in the rate of Mn2+ entry (P = 0.02), whereas there was no significant increase following thapsigargin stimulation of differentiated cells (P = 0.62). This suggests that whilst in control cells there is a functional CCE pathway that can be stimulated by thapsigargin, in cells differentiated with 9cRA this pathway plays no significant role, and may be shut off or down-regulated.
We are currently investigating whether activation of a CCE pathway such as this could account for the differences in response to methacholine stimulation seen in control cells compared with that seen in 9cRA-differentiated cells.
This work is supported by a Programme Grant from the MRC to T.R.C. and a project grant from the NECCRF.