Background: The human respiratory epithelium is lined with hundreds of millions of motile cilia that beat in a coordinated fashion to clear mucus, pathogens, and pollutants from the airways. Several diseases cause abnormal ciliary function, including severe asthma, COPD, cystic fibrosis and primary ciliary dyskinesia (PCD) (1). PCD is an autosomal recessive disorder that affects around 1:8000 new-born babies. Mutations in ciliary genes lead to protein defects within the cilia that cause them to beat in a dyskinetic fashion or become static. PCD patients demonstrate reduced mucociliary clearance, recurrent respiratory tract infections and progressive severe lung damage. However, our understanding of the epithelial biology of PCD patients remains incomplete (2).
Aims:
- To determine if the cellular composition of ciliated epithelial cell cultures from PCD patients is similar to that of matched healthy controls.
- To determine if baseline cytokine and chemokine release from PCD and healthy control cultures differ.
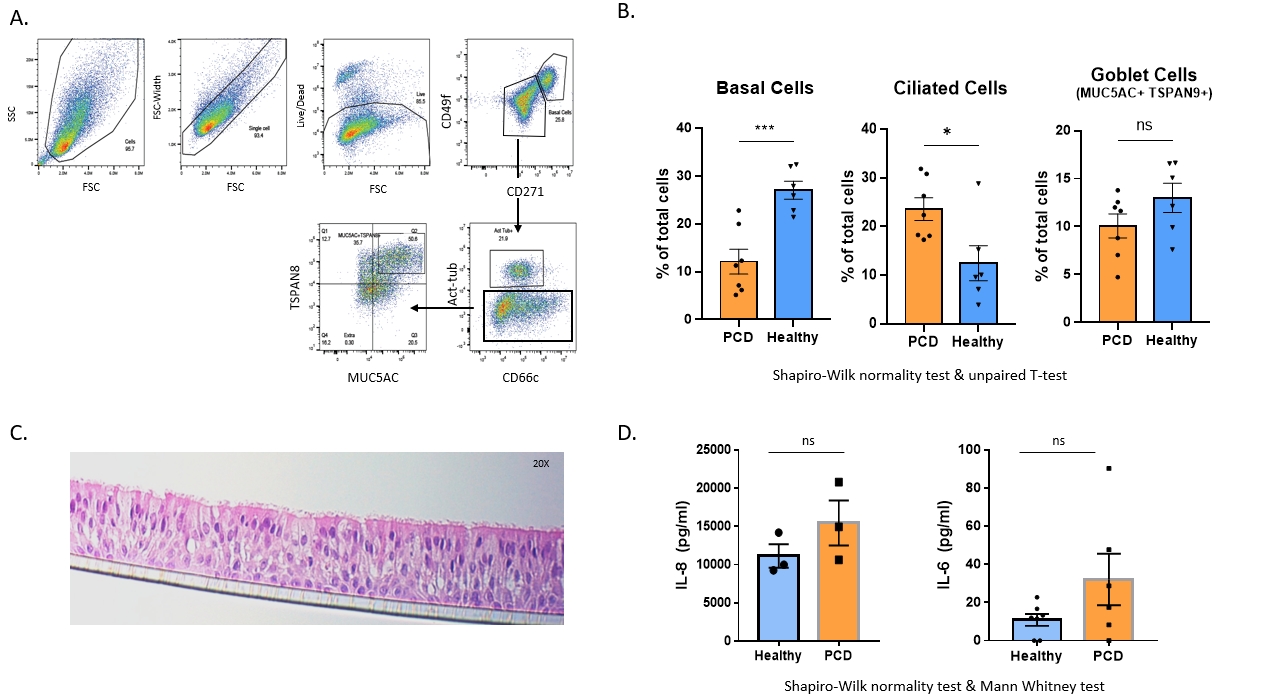
Methods: Nasal brush biopsies from PCD patients with static cilia were cultured at air-liquid interface (ALI) to a ciliated phenotype (3). To characterise the cellular composition, we optimised a flow cytometry panel adapted from Bonser et.al, to quantify three major epithelial cell types: basal, ciliated and goblet cells (4). We used a histopathology approach, sectioning the ALI cultures to visualise epithelial populations using H&E (Figure 1). Finally, supernatants were analysed for cytokine changes using ELISA. Static PCD nasal brushings were age/sex matched with healthy controls. The mean and S.E.M was calculated, and statistical significance determined by firstly testing the normality of data using Shapiro Wilks test, followed by two-tailed T-test (flow cytometry) or non-parametric Mann Whitney test (ELISA).
Results: We confirmed the epithelial morphology of ALI is maintained during histology processing and H&E-staining (n=6 Healthy & n=PCD, 4 sections/donor). Flow cytometry showed PCD patients had fewer basal cells (n = 6 healthy, 7 PCD; p =0.0009) and an increased number of ciliated cells (p =0.0221) compared to age/sex match controls. Initial studies suggest a trend for increased IL-8 (n=3) and IL-6 (n=6) levels in PCD supernatants however increased donor number is required for statistical significance.
Summary: Flow cytometry showed a difference in the cellular composition of PCD ALI cultures, with increased numbers of ciliated cells and reduced numbers of basal cells. Preliminary ELISA results suggest static PCD may be associated with increased epithelial inflammation. Further characterisation is underway, increasing study numbers and processing cultured cells for RNAseq and mass spec to investigate apical secretions. Overall, this work will improve our basic understanding of PCD epithelial biology and identify potential PCD-specific therapeutic targets.