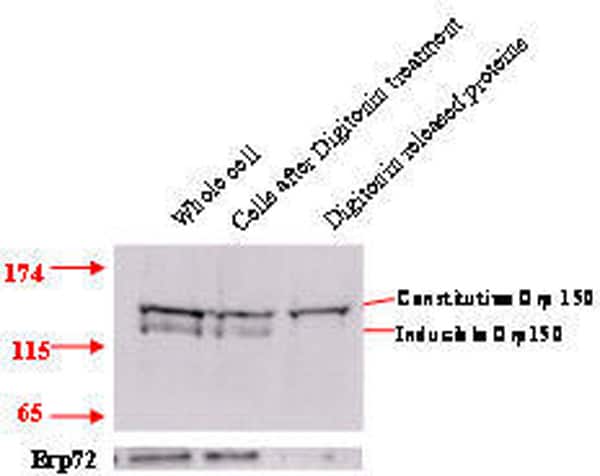
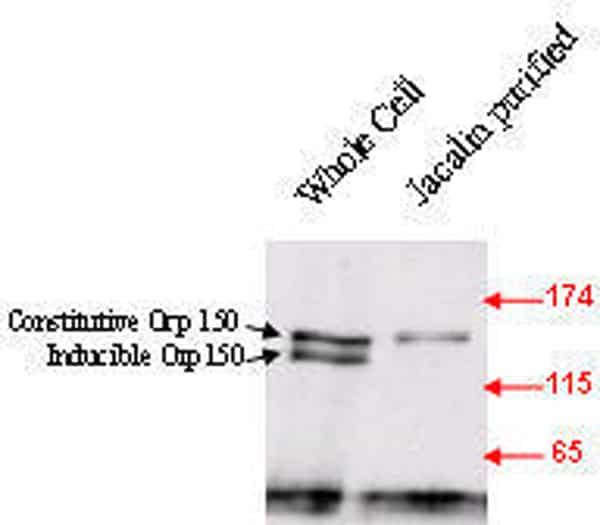
Changes of cellular glycosylation, such as the increased expression of the oncofetal Thomsen-Friedenreich antigen (galactoseβ1,3N-acetylgalactosamineα-,TF), are common in cancer and pre-cancerous conditions(Campbell, et al 2001) . We have previously shown that lectins that bind TF can have marked effects on proliferation. The edible mushroom (Agaricus bisporus) lectin (ABL) has an anti-proliferative effect(Yu et al 1993) that relates to its internalisation and inhibition of NLS-dependent nuclear protein import mediated via its interaction with an N-terminally truncated form of the stress glycoprotein Orp150 (Yu et al, 2002). The aim of the present study is to characterise Orp150 glycosylation and investigate further the role of Orp150 in nuclear protein import. Immunoblotting of protein extracts from HT29 colon cancer cells showed the presence of two Orp150 isoforms of which the lower molecular weight isoform is stress inducible by glucose starvation. The higher molecular weight isoform is constitutively expressed. Selective membrane permeabilisation with digitonin showed release of only the constitutive Orp150 form implying its cytoplasmic localisation (Fig 1). Lectin affinity purification of protein extracts from a glucose starved cell preparation with Jacalin (a TF and sialyl-TF-binding lectin from jack fruit) yielded selective extraction of the constitutive Orp150 form suggesting differential glycosylation of the two Orp150 isoforms (Fig 2). siRNA treatment of HT29 cells resulted in 62% reduction of Orp150 expression and subsequently 89% reduction (% nuclear fluorescence, without heat stress 53.97, after heat stress 75.44, siOrp150 + heat stress 56.26, P<0.0001 ) of the nuclear translocation of Hsp70 in response to heat stress, thus confirming a role for Orp150 in NLS-dependent nuclear protein import. Further studies will investigate the possible role of O-glycosylation in Orp150 function.This study suggests that there are two Orp150 isoforms, one is a truncated cytosolic form and the other is a stress inducible form. The cytosolic form is involved in NLS-dependent nuclear protein import.
Life Sciences 2007 (2007) Proc Life Sciences, PC315
Poster Communications: Characterization of glycoforms of the stress-related Orp150 involved in NLS-dependent nuclear protein import
B. A. Tam1, J. M. Rhodes1, L. Yu1
1. Gastroenerology, University of Liverpool, Liverpool, United Kingdom.
View other abstracts by:
Fig 1. Orp150 immunoblot of HT29 cells shows selective release of constitutive but not the inducible Orp150 form by digitonin permeabilisation. Erp72 is an ER localised protein. Fig 2. Jacalin affinity purification of glucose starved HT29 cell preparation followed by Orp150 immunoblot shows extraction of the constitutive Orp150 isoform.
Fig 1. Orp150 immunoblot of HT29 cells shows selective release of constitutive but not the inducible Orp150 form by digitonin permeabilisation. Erp72 is an ER localised protein. Fig 2. Jacalin affinity purification of glucose starved HT29 cell preparation followed by Orp150 immunoblot shows extraction of the constitutive Orp150 isoform.
Where applicable, experiments conform with Society ethical requirements.