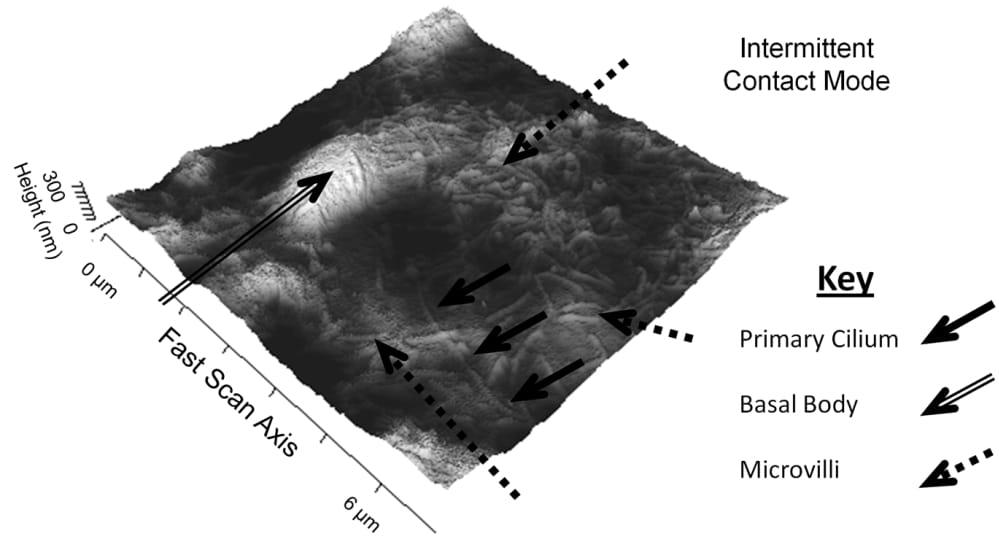
Primary cilia are non-motile specialised mechano-sensory organelles that protrude from the surface of epithelial cells. In the kidney, they are 200 nm in diameter and extend approximately 10 µm into the lumen of the nephron. Ciliary dysfunction is linked to autosomal dominant polycystic kidney disease, the most common of the inherited cystic diseases and thus, understanding the functioning of the cilium and the mechano-sensitive channels thereon is crucial to furthering research into the disease. The manner in which the cilium bends in response to fluid flow along the nephron and the mechanisms leading to the intracellular calcium release are widely debated. Here, an interdisciplinary approach has been adopted to investigate the biophysical properties of the primary cilium to gain insight into ciliary bending. Initially, fixed cells were imaged by Atomic Force Microscopy (AFM) in Intermittent Contact Mode (Figure 1) to confirm MDCK type II cells were manifesting primary cilia. On unfixed cell layers, a TCS SP5 Confocal Microscope (Leica, Wetzlar, Germany) was then used to identify cells with a primary cilium and then position the cantilever of the AFM instrument (JPK, Berlin, Germany) in the near vicinity of the cilium. We controllably probed individual primary cilia with the AFM cantilever tip with forces of up to 13.5 pN to obtain a vertical deflection map in one plane. By probing primary cilia at different heights from the surface of the cell, we produce AFM “deflection volume” data sets. This detailed probing of the mechanical response of the cilium as a function of height is a mechanical analogue of the z-stack optical imaging employed in confocal microscopy. Using this approach, we have measured the stiffness of the cilia, with spring constants of 4 ± 2 x 10-5 Nm-1 (mean ± S.E.M, n=7) consistent with other techniques (1, 2). This result supports the hypotheses of Schwartz et al. (1) and Resnick (2), in that the primary cilium bends due to mechanical strains of the order of 10-5 Nm-1 and hence is able to act as a flow sensor in the kidney.
Durham University (2010) Proc Physiol Soc 21, C12 and PC12
Oral Communications: Combining Scanning Probe and Confocal Microscopy: A new method for studying the Primary Cilium
S. Kocher1, D. N. Sheppard2, T. J. McMaster1
1. School of Physics, University of Bristol, Bristol, United Kingdom. 2. School of Physiology and Pharmacology, University of Bristol, Bristol, United Kingdom.
View other abstracts by:
Figure 1: An AFM image of the apical surface of an MDCK Type II cell. Lighter colours indicate higher parts of the sample. A primary cilium (solid arrows) can be seen extending from the basal body (hollow arrow). The remaining hair-like structures on the surface are microvilli (dashed arrows).
Where applicable, experiments conform with Society ethical requirements.