The role of the sarcoplasmic reticulum (SR) during excitation-contraction (E-C) coupling in trout ventricle is unclear. Similar to amphibians, trout ventricular myocytes are long and thin, devoid of t-tubules and rely primarily on extracellular Ca2+ entry to initiate myofilament contraction. Similar to mammals, trout myocytes have a fairly well developed SR, capable of accumulating large Ca2+ stores which can contribute to E-C coupling under certain conditions.
Our aim in this study was to better understand the role of the trout SR during E-C coupling by investigating temporal and spatial co-ordination of the Ca2+ transient and the occurrence of Ca2+ sparks in intact, contracting trout ventricular myocytes using confocal microscopy. Ca2+ sparks and Ca2+ transients have not been reported for fish hearts. Therefore, in addition to trout, experiments were conducted on rat ventricular myocytes as a positive control.
Wistar rats and rainbow trout were humanely killed. Myocytes from both species were loaded with 4-10 µM Fluo 4 and were examined with repetitive line scans (1000 lines of 512 pixels, 4-7 ms intervals) across the width of the cell. All values are means ± S.E.M. Differences were considered significant at P < 0.05 as assessed with Student’s unpaired t test and RM ANOVA with SNK post hoc.
In contrast to de-tubulated rat ventricular myocytes which show a pronounced delay (>100 ms) of initiation in the Ca2+ wave-front in the centre of the cell, trout myocytes show a delay of only ~8 ms between cell periphery and cell centre. Additionally, the time to peak of transient was longer (P = 0.034, n = 7) and the rate of rise was slower (P = 0.016, n = 7) in the centre versus the periphery of the trout myocyte.
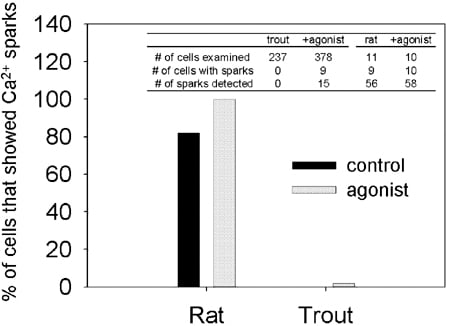
Spark-like events were observed in only a very small number of the trout myocytes and then only after agonist stimulation (Fig. 1). Decreasing temperature from 21 °C to 14 °C had no effect on spark frequency. In general, trout ventricular spark-like events were narrower and shorter, with faster rise and 50 % decay times than those observed in rat (P < 0.05).
In summary, in trout ventricular myocytes, the Ca2+ wave-front is initiated at the cell periphery and diffuses rapidly to the cell centre probably due to the narrow width (8.1 ± 1.9 µm) and depth (6.5 ± 0.5 µm) of the cell. Ca2+ spark-like events were not found under control conditions which may relate to the density or type of ryanodine receptor present.
This work was supported by the BHF (E.W.) and NSERC Canada (H.S.).