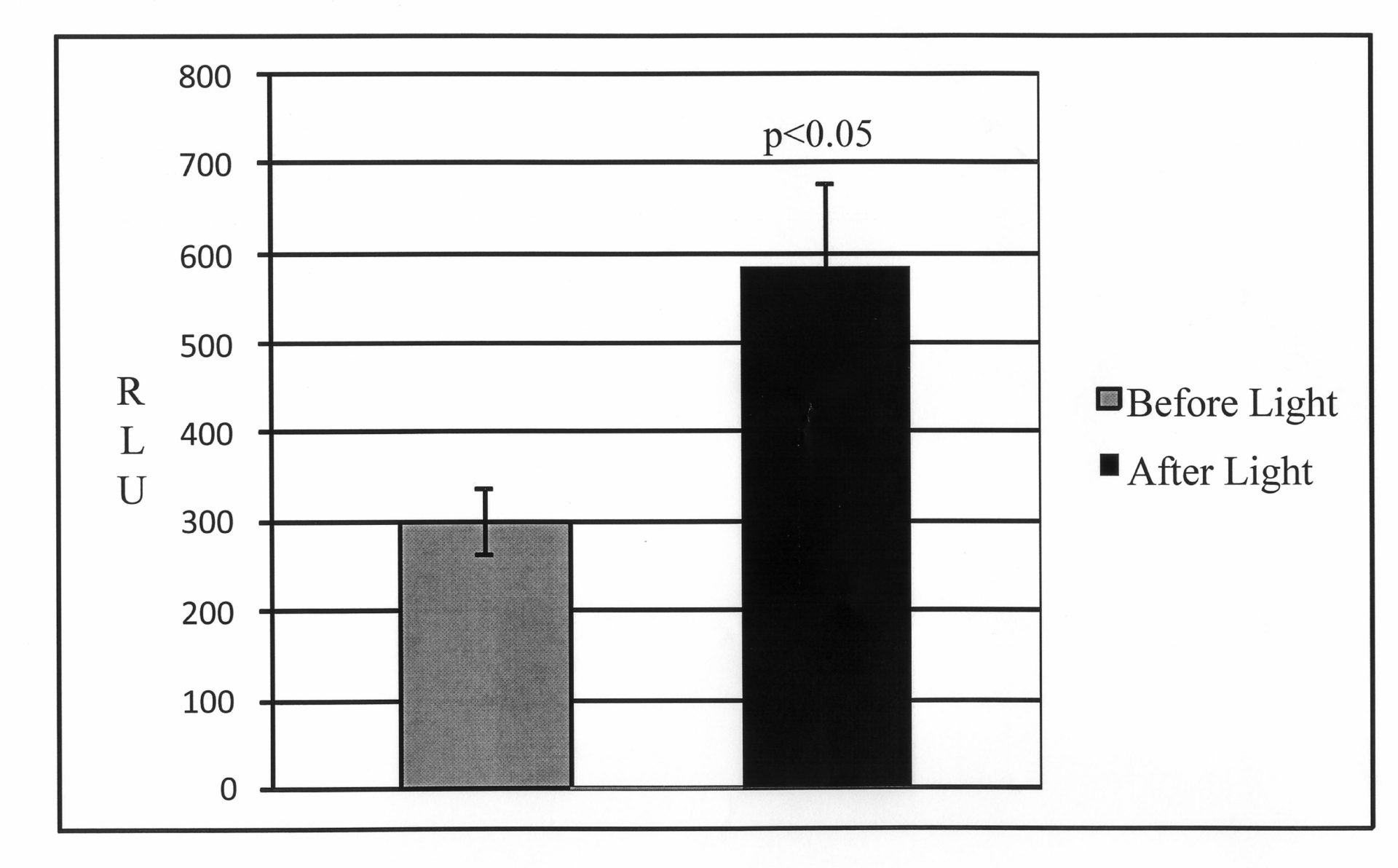
Astrocytes are an integral part of the brain, where they form connections with blood vessels, neurones and other glia. ATP is considered to be the major gliotransmitter, but the mechanisms which control its release are not completely understood. According to earlier work (1), ATP release is at least partially a vesicular, Ca2+ -dependent process. We used optogenetics in order to trigger intracellular Ca2+([Ca2+]i) increase selectively in astrocytes. We have developed a glia-specific adenoviral vector which employs a shortened glial fibrillary acidic protein (sGFAP) promoter and a bidirectional transcriptional amplification strategy to enhance its transcriptional activity (2). The vector expresses a mutated version of the algal light sensitive non-selective cation channelrhodopsin-2, ChR2(H134R) (3), fused to a novel fluorescent protein Katushka1.3 which has its emission peak at ~630 nm (4). Katushka1.3 can be excited by green or yellow laser light, thus avoiding activation of ChR2(H134R) by blue light. Characterization of the new vector, AVV-sGFAP-ChR2(H134R)-Katushka1.3, was performed in primary cultures of rat astrocytes. [Ca2+]i in Rhod-2-AM loaded astrocytes was monitored using a SP1 Leica confocal microscope specifically modified for that purpose. Astrocytes were continuously perfused with Hank’s Balanced Salt Solution (HBSS) at 34oC. Flashing blue light from a LED source (470 nm; 20/20 ms duty cycle) triggered robust increases (19.3%; n=12; p<0.001; Student’s paired t-test) in [Ca2+]i in all transduced primary astrocytes. In order to test whether these increases depend on the influx of Ca2+ from the extracellular space, astrocytes were superfused with Ca2+ -free HBSS with EDTA for 30 minutes. This resulted in almost complete elimination of light-induced [Ca2+]i increases. To test whether the new vector can be used to trigger release of ATP in a more integrated preparation, organotypic slices of rat pups (p8-p10) were prepared as previously described (5) and transduced with AVV-sGFAP-ChR2(H134R)-Katushka1.3. After 7 days, slices were placed into a tissue chamber and superfused with HBSS at 34oC at 0.5 ml/min. ATP levels in the outflow were measured using a luminometer (Berthold Technologies; Lumat LB9507) and an ATP Bioluminescent assay kit according to the manufacturer’s instructions (Sigma Aldrich). Blue light (445 nm wavelength ~7 mW/mm2) applied to the transduced slice evoked a +95% increase in ATP release (n=5; p<0.05; Student’s paired t-test). These data demonstrate that optogenetically stimulated astrocytes respond with an increase in [Ca2+]i which is largely coming from the extracellular space, and with the release of ATP. Optogenetic stimulation of astrocytes opens a wide range of opportunities for enquiries into the physiology and signalling properties of these cells.
University of Manchester (2010) Proc Physiol Soc 19, C21
Oral Communications: Control of astrocytic ATP release using optogenetics
M. Figueiredo1, F. Tang1, A. Gourine2, A. G. Teschemacher1, S. Kasparov1
1. Physiology-Pharmacology, University of Bristol, Bristol, United Kingdom. 2. Neuroscience, University College London, London, United Kingdom.
View other abstracts by:
Figure 1. Light activation of astrocytes transduced with AVV-sGFAP-ChR2(H134R)-Katushka1.3 increases ATP release.
Where applicable, experiments conform with Society ethical requirements.