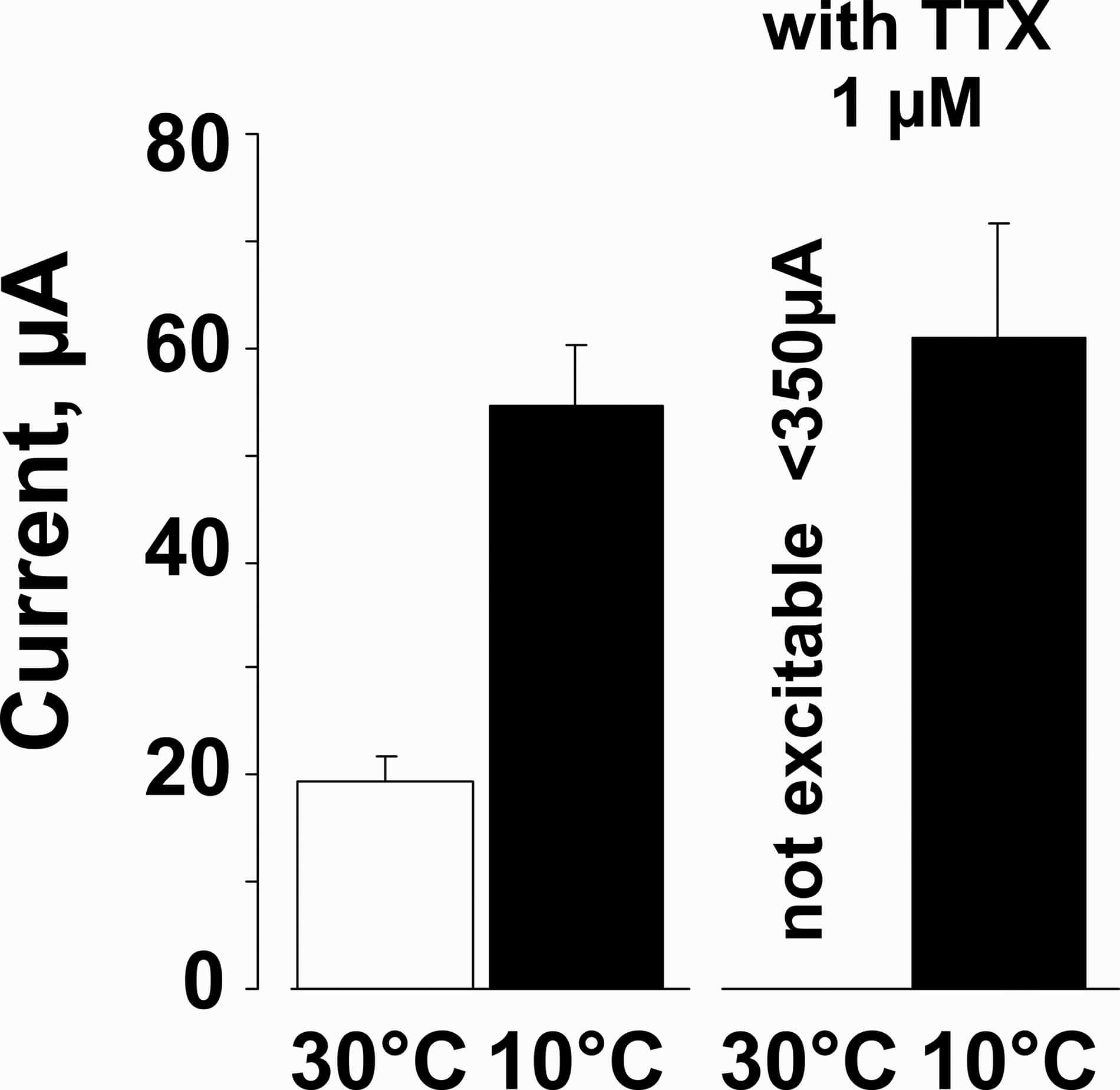
Sensory transduction and subsequent impulse generation are processes in the sensory neuron that involve ion channels. Sensory transduction is specific for the quality of the stimulus, and composed of inward and outward current components contributed by cation channels of the transient receptor potential subfamily (TRP), e.g. TRPM81 and TRPA12, and potassium channels3, respectively. Transduction depolarizes the nerve terminal to action potential threshold and subsequent impulse generation involves voltage-gated sodium channels (VGSC). When temperatures get colder, the kinetics of all channels slow down which goes along with a deterioration of sensory acuity and motor dexterity. Nevertheless, the detection of noxious cold is not impaired and pain in the cold is perceived even more intensely. Evolutionary pressure to enforce protective behaviour against cold damage requires nociceptors to continue to function at low temperatures. To ensure impulse generation the superficial endings of slowly conducting cutaneous nociceptive C-fibers are endowed with different VGSC α-subunits that exhibit fast (e.g. Nav1.7) or slow (Nav1.8 and Nav1.9) kinetics. Whereas the fast-gated VGSC are selectively blocked by tetrodotoxin (TTXs), both Nav1.8 and Nav1.9 are TTX-resistant. Nav1.8 is expressed exclusively in sensory neurons4. It generates a slowly-inactivating current with a high threshold for activation and is the only VGSC that is able to generate action potentials in the presence of TTX. Measuring sodium channel activity reveals that under cold conditions the availability of sodium current through fast VGSC subunits is dramatically reduced due to slow inactivation. Yet, the inactivation properties of the TTXr channel Nav1.8 are entirely cold resistant. If Nav1.8 is deleted, mouse cultured sensory neurons turn unexcitable at cold temperatures5; however, the cutaneous terminals are able to sustain excitability to mechanical and electrical threshold stimuli which is achieved by compensatory overexpression/alteration of TTXs fast-gated VGSC6. Strongly reduced, however, is the sensitivity of Nav1.8 deficient nerve endings to noxious mechanical and cold stimulation. Thus, in the absence of Nav1.8, menthol does not exert its usual effect of sensitizing to cold, but its action at normal skin temperature (30°C) remains unchanged. This shows that transduction and electrogenesis are separate processes; while transduction by cold/menthol is unchanged in cutaneous terminals of Nav1.8-/- neurons, electrogenesis in the cold is strongly impaired. Puzzling is the finding that much smaller currents are required in the cold than at 30°C to trigger action potentials through Nav1.8 in wildtype cutaneous terminals stimulated in the presence of TTX (Fig.). Measuring the electrical strength-duration relationship in terminals allowed assessing cold-induced changes in passive and active membrane properties. Cooling caused an increase in the current threshold at shorter stimulus durations and a prolongation of the chronaxy, the most efficient stimulus duration in terms of charge transfer. Both effects reflect the slowing of voltage-gated sodium channel kinetics in the cold. TTX increased both the current threshold at all stimulus durations and the chronaxy which is consistent with the high threshold and slow kinetics of Nav1.8. Cooling, in presence of TTX, decreased the current thresholds at all stimulus durations including chronaxy, reflecting an increase in input membrane resistance that may be of particular importance in nerve endings with their high ratio of surface area to volume. The resulting reduction of leak conductance augments any voltage change caused by depolarizing currents and, thus, facilitates reaching the high threshold of Nav1.8 when Nav1.7 undergoes cold block. In addition, nerve endings depolarize due to a cold-induced block of the sodium-potassium ATP-ase, and a closure of certain potassium channels belonging to the group of 2-pore channels (2pK), namely TREK1, TREK2 and TRAAK which are temperature sensitive and expressed in sensory nerve terminals7. At body temperature 2pK channels are open, stabilizing the resting membrane potential, but under cold conditions their closure leads to an increase in excitability of the nerve endings. Consistent with this concept, mice deficient of both TREK1 and TRAAK show a remarkable hypersensitivity to cold demonstrated by both an increase of the prevalence of cold nociceptive fibers and of their cold response3. Nav1.8-null mice, on the contrary, show negligible responses to noxious cold. Taken together, the high threshold for voltage-dependent fast inactivation renders Nav1.8 tolerant of tonic depolarization and, due to lack of slow inactivation under cold conditions it remains available as the sole electrical impulse generator in nociceptors that transmit information to the central nervous system.
University College Dublin (2009) Proc Physiol Soc 15, SA11
Research Symposium: Cool, Cold or Painfully Cold? – How cold-sensing ion channels create temperature sensations.
K. Zimmermann1
1. Dept. of Physiology and Pathophysiology, Medical Faculty University Erlangen-Nürnberg, Erlangen, Bavaria, Germany.
View other abstracts by:
TTX blocks excitability of nociceptor nerve endings, and cold temperatures reverse the TTX block. Electrical thresholds of saphenous nerve C-fiber terminals at 30°C (open bars) and 10°C (filled bars) before and in the presence of TTX. Cooling doubled thresholds for electrical excitation. Application of TTX blocked the units at 30°C but cooling to 10°C recovered excitability.
Where applicable, experiments conform with Society ethical requirements.