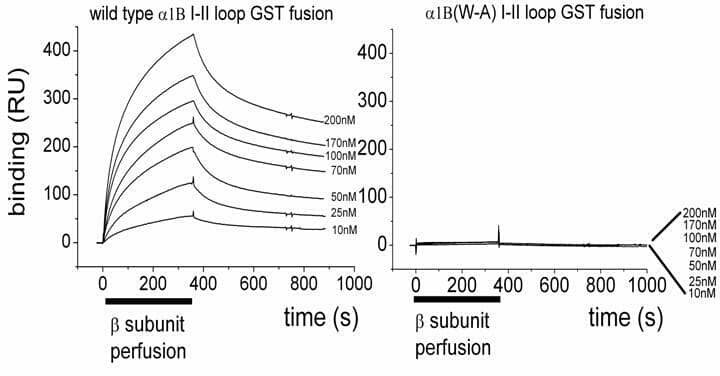
High-voltage activated calcium channels (HVA) are heteromultimers composed of a main pore-forming Cavα1 subunit associated with auxiliary subunits β and α2δ. The β subunits modulate biophysical properties of the channels and promote the voltage-dependence of modulation of N-type calcium channels by G proteins (Meir et al., 2000). They bind to an interaction site of nine amino-acids (QQxExxLxGYxxWIxxxE), the Alpha-Interaction Domain (AID), conserved in the I-II loop of the Cavα1 subunit of all HVA calcium channels. By mutating W391 to A in the AID of Cav2.2, we confirmed that this amino-acid is essential for β1b subunit binding (Fig. 1), for plasma membrane expression and for modulation of the biophysical properties of CaV2.2 channels. Whereas robust currents were recorded co-expressing Cav2.2 with β1b (−219.47±41 pA/pF at +20 mV ; (mean±S.E.M, n=12), co-transfection of Cav2.2(W391A) with β1b induced small currents (−31.75±7.4 pA/pF at +20 mV; n=11). Moreover, without β, Cav2.2 produced small but recordable currents (−8.22±1.1 pA/pF at +20 mV ; n=13) while the currents were negligible when Cav2.2(W391A) was expressed alone (n=21), suggesting that some endogenous β may be present in tsA-201 cells and trafficking wild type Cav2.2. Activating with 100 nM quinpirole a Dopamine D2 receptor co-transfected in tsA-201 cells with Cav2.2, β1b and α2δ2 allowed us to study the G protein modulation of N-type calcium channels. For the wild type channel, quinpirole induced an inhibition of 53.5±3.8 % (n=12) of the recorded currents and a +7 mV shift of its activation. Time constant of activation (Tau) was slowed from 2.45±0.55 ms to 4.64±0.48 ms and prepulse potentiation was observed. The Cav2.2(W391A) mutant was still inhibited (46.4±8 % n=11) by G-proteins and a positive shift of +6.5 mV for its activation still occurred but the slowed activation kinetics and prepulse potentiation were not observed for this mutant. However, in contrast to expectation from the crystal structure, mutation of another amino-acid within the AID, Y388 to S did not affect channel properties or its G-protein modulation. These results confirm that the W391 is crucial for β subunits binding to the I-II linker of Cav2.2, and show that no Cav2.2 is functional at the plasma membrane in the complete absence of interaction with a β subunit. The results also provide evidence for the role of β subunits in the voltage-dependence of modulation of N-type calcium channels by G protein-coupled receptors.
King's College London (2005) J Physiol 565P, C82
Communications: CRUCIAL ROLE OF BETA SUBUNITS FOR EXPRESSION AND G PROTEIN MODULATION OF N-TYPE CALCIUM CHANNELS REVEALED BY MUTATIONAL ANALYSIS OF THE I-II LOOP OF Cav2.2
Leroy, Jerome ; Richards, Mark ; Butcher, Adrian J ; Nieto-Rostro, Manuela ; Pratt, Wendy S ; Davies, Tony ; DOLPHIN, Annette C;
1. Pharmacology, University College London, LONDON, LONDON, United Kingdom.
View other abstracts by:
Figure 1: Examples of Biacore 2000 sensorgrams for comparison of purified Beta1b binding to a GST-Cavv2.2(W391A) I-II loop (right).
Where applicable, experiments conform with Society ethical requirements.