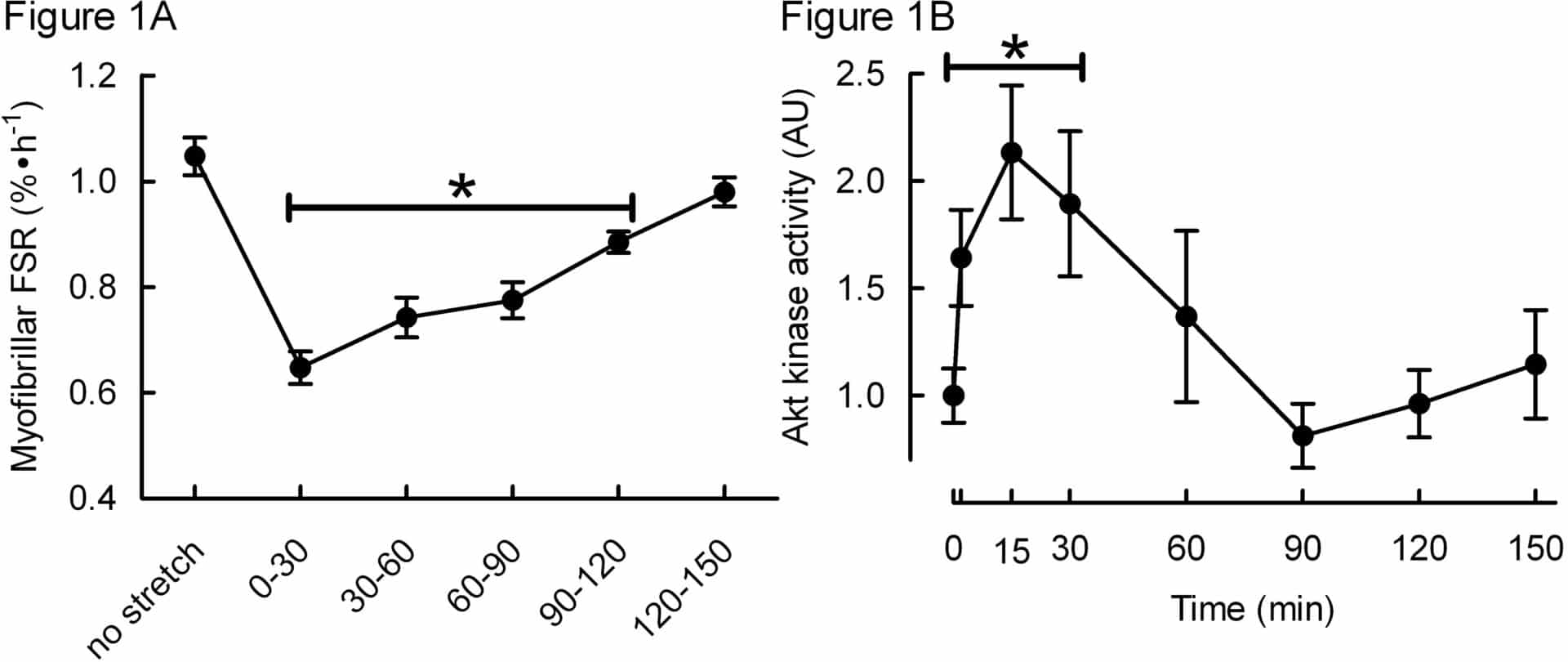
Muscle protein synthesis is modulated by mechanical stimuli: during strenuous muscular activity, protein synthesis is suppressed (Rose et al., 2009) but increases afterwards, resulting in a net increase of protein if sufficient amino acids are available (Rennie and Tipton, 2000). In life, muscles are subjected to shortening forces due to contraction, but are also subject to stretching forces during lengthening. It would be inefficient if contraction and stretch had different effects on muscle protein turnover, but little is known about the topic. To investigate this, we measured myofibrillar and sarcoplasmic protein synthesis (MPS, SPS, respectively) by incorporation of 13C proline using gas chromatography-mass spectrometry and anabolic signalling by phospho-immunoblotting and kinase assays in cultured L6 skeletal muscle cells. Aside for unstretched controls, all cells were cyclically stretched using a 1Hz sine wave for 30 min and sampled at 2, 15 and 30 min and over 30 min intervals up to 120 min after stopping stretch (n=6 technical replicates/condition). One-way ANOVA with Tukey’s post-hoc testing was used to assess differences in protein synthesis and signalling between stretched and unstretched cells; data are presented as mean±SEM where significance was set at P<0.05. During stretch, SPS was unaffected, whereas MPS was suppressed by 40±0.03% (P<0.01; see Figure 1A) before returning to basal rates 90-120 min after stopping stretch. Paradoxically, anabolic signalling was stimulated with peak values after 2-30 min: e.g. focal adhesion kinase (FAK Tyr576/577; +28±6%), protein kinase B activity (Akt; +113±31%; see Figure 1B), p70S6K1 (ribosomal S6 kinase Thr389; 25±5%), 4E binding protein 1 (4EBP1 Thr37/46; +14±3%), eukaryotic elongation factor 2 (eEF2 Thr56; -47±4%), and extracellular regulated protein kinase 1/2 (ERK1/2 Tyr202/204; +65%±9%); all P<0.05. After stopping stretch, except for Akt activity, stimulatory phosphorylations were sustained: e.g. FAK (+26±11%) for ≥30 min, eEF2 for ≥60 min (peak -45±4%), 4EBP1 for ≥90 min (peak +33±5%), and p70S6K1 which remained elevated throughout (peak +64±7%); all P<0.05. Neither AMPK (Adenosine monophosphate-activated protein kinase) Thr172 or ACCβ (acetyl-CoA carboxylase) Ser79 phosphorylation were affected by stretch. Thus acute cyclic stretch specifically suppresses MPS despite paradoxical increases in activity/phosphorylation of elements thought to increase anabolism (i.e. Akt-mTORC1 signalling). Given the absence of inhibitory Ca2+-eEF2K-eEF2 (Rose et al., 2009) and AMPK signalling, we propose the existence of other mechanisms regulating the inhibition of muscle protein synthesis induced by mechanical activity.
King's College London (2009) Proc Physiol Soc 14, C5
Oral Communications: Cyclic stretch reduces myofibrillar protein synthesis despite increases in Focal Adhesion Kinase and anabolic signalling in L6 cells
P. J. Atherton1, N. J. Szewczyk1, A. Selby1, D. Rankin1, K. Hillier2, K. Smith1, M. J. Rennie1, P. Loughna2
1. Clinical Physiology, University of Nottingham, Derby, United Kingdom. 2. School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonnington, United Kingdom.
View other abstracts by:
*Asterisks over bars indicate significantly different (P<0.05) from no stretch conditions.
Where applicable, experiments conform with Society ethical requirements.