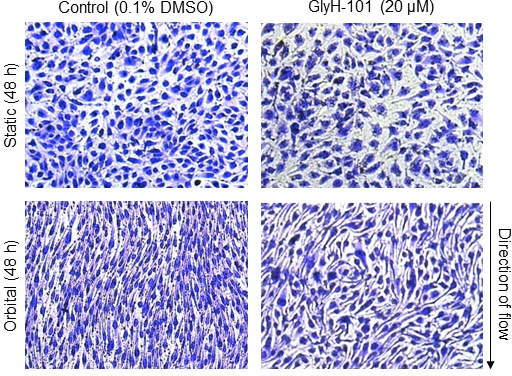
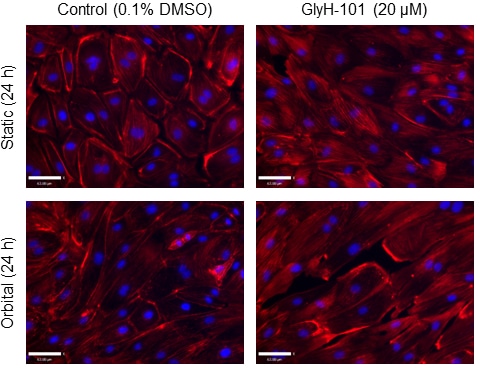
Problem statement: Flow mediated dilation is compromised in cystic fibrosis (CF), demonstrating endothelial dysfunction in response to shear stress. Inhibition of endothelial CF-transmembrane conductance regulator (CFTR) activity causes a pro-inflammatory and pro-oxidant state. Inflammation and oxidative stress cause endothelial barrier dysfunction by re-organising the endothelial actin cytoskeleton. It is currently unknown, however, whether CFTR inhibition causes F-actin polymerisation. The present study, therefore, aimed to investigate the implications of CFTR inhibition on F-actin polymerisation and morphological alignment following exposure to shear stress in human lung microvascular endothelial cells (HLMVEC) in vitro. Methods: HLMVEC were cultured in the absence and presence of the pharmacological CFTR inhibitor GlyH-101 (20 µM), during 16 h, 24 h and 48 h of exposure to shear stress (11.1 dynes/cm2) or static conditions. Nitrite and endothelin (ET)-1 were analysed in cell media supernatants. Morphological changes in response to shear stress were detected using Crystal Violet staining. Cortical rim, perinuclear and stress fibre F-actin reformation were detected using Rhodamine Phalloidin (red) and Hoechst 33342 (blue) staining. Values are means ± SEM. Results: Exposure to 48 h of shear stress increased [nitrite] (+146.2 ± 2.0% vs. static conditions; p < 0.05), whereas 48 h (-57.9 ± 3.6% vs. static conditions; p < 0.05) and 24 h (-63.5 ± 5.6% vs. static conditions; p < 0.05) of shear stress lowered [ET-1] in HLMVEC supernatant. Notably, 16 h or 24 h of shear stress had no significant effect on [nitrite], nor did 16 h of shear stress significantly affect [ET-1] (p > 0.05). The addition of GlyH-101 did not alter [nitrite] or [ET-1] (all p > 0.05). Shear stress over 48 h induced cellular alignment with the direction of flow; however, the addition of GlyH-101 prevented such alignment (Figure 1). GlyH-101 increased cytosolic F-actin polymerisation under static conditions (p < 0.05). GlyH-101 also caused a re-distribution of F-actin from the cortical rim to cytosolic stress fibres under both static and shear stress conditions over 24 h (Figure 2). Conclusions: Functional CFTR appears to limit cytosolic F-actin polymerisation, while maintaining a cortical rim distribution that is important for cellular integrity and CFTR function. Inhibition of CFTR, with increased F-actin stress fibre formation, prevented HLMVEC alignment with flow independent of [nitrite] and [ET-1]. CFTR, therefore, plays an important role in the regulation of actin dynamics in HLMVEC and may contribute to the endothelial perturbations that are evident even in relatively mild CF.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, C009
Oral Communications: Cystic fibrosis-transmembrane conductance regulator limits F-actin formation and promotes morphological alignment with flow in human lung microvascular endothelial cells.
A. J. Causer1, M. Khalaf2, E. Rote2, K. Brand2, S. Bailey3, M. Cummings4, A. Shepherd1, Z. L. Saynor1, J. Shute2
1. Department of Sport & Exercise Science, University of Portsmouth, Portsmouth, United Kingdom. 2. School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth, United Kingdom. 3. School of Sport, Exercise and Health Sciences, Loughborough University, Loughborough, United Kingdom. 4. Department of Diabetes and Endocrinology, Queen Alexandra Hospital, Portsmouth, United Kingdom.
View other abstracts by:
Figure 1. Crystal violet staining of human lung microvascular endothelial cells cultured in the absence (Column 1) or presence (Column 2) of GlyH-101, following 48 h of static incubation (Row 1) or 11.1 dynes/cm2 of shear stress (Row 2).
Figure 2. F-actin staining of human lung microvascular endothelial cells cultured in the absence (Column 1) or presence (Column 2) of GlyH-101, following 24 h of static incubation (Row 1) or 11.1 dynes/cm2 of shear stress (Row 2).
Where applicable, experiments conform with Society ethical requirements.