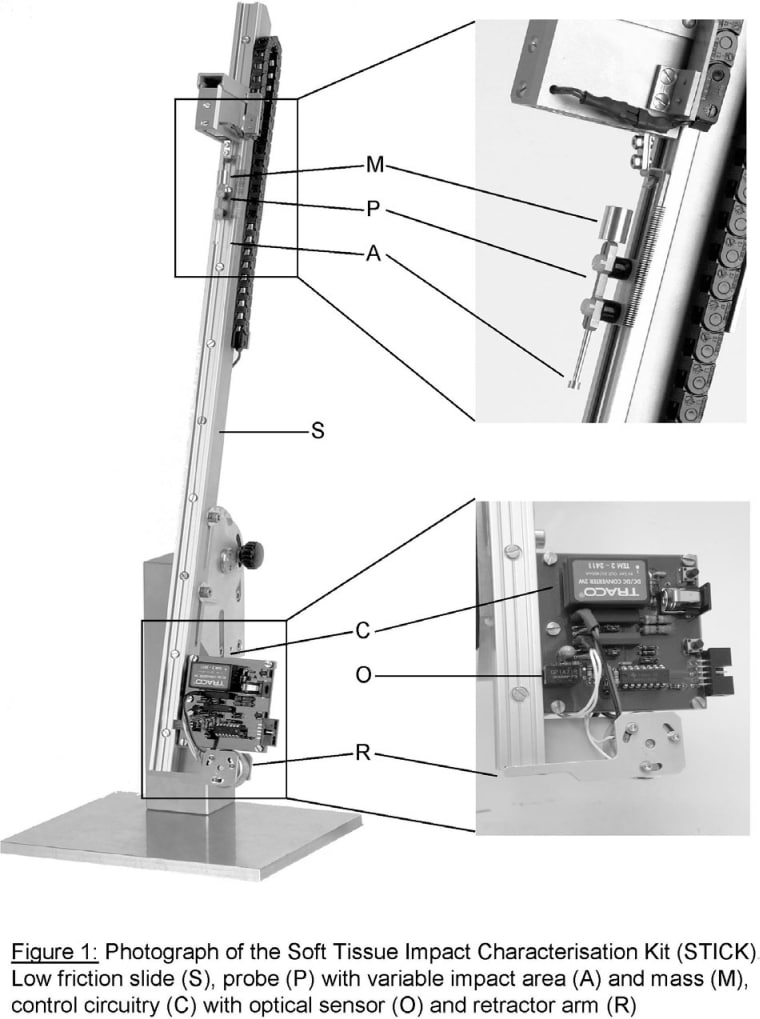
Commotio cordis [shaking of the heart] refers to mechanically induced disturbances in heart rhythm, usually brought about by impacts to the pre-cordial chest. In contrast to contusio cordis [bruising of the heart], commotio is not accompanied by structural damage to the chest and its organs (in particular the heart) that could explain the observed electrophysiological effects. Case registries, whole animal experiments, and theoretical considerations have identified a number of risk factors and probable mechanisms (reviewed in [1]). Currently, there is no isolated heart model of the condition, which makes it difficult to assess (sub-)cellular mechanisms. Even the actual energy threshold at which commotio turns into contusio cordis (i.e. when tissue damage occurs) is, as yet, unknown. A previous report suggested that impacts of up to 200 mJ cause only superficial tissue damage in rabbit isolated heart [2]. This study is designed to assess that suggestion.Mechanical impacts were performed using a custom-built device [3], comprising a low-friction sliding mechanism that carries a probe of variable weight and impact surface area (Fig. 1). The probe is released from a user-defined height (triggered manually or by computer), and its position immediately prior to and during impact is measured using an optical grid system (resolution < 0.2 mm), interfaced with a BioPac MP150 (200 kHz per channel), and AcqKnowledge software. Upon complete deceleration, a hardware-controlled retractor arm swiftly removes the probe from the tissue to prevent secondary interaction. Based on known probe mass and impact area, and on measured pre-impact speed and deceleration characteristics, we obtain precise data on parameters such as force, pressure, deformation and work.Impacts were targeted at the left-ventricular free wall of isolated, Langendorff-perfused hearts from Guinea pigs (killed by cervical dislocation; all investigations conform with UK Home Office Regulations). Hearts were positioned in a cradle underneath the impactor and perfused with either Krebs-Henseleit (KH) solution (in mM: NaCl 118, CaCl2 1.8, KCl 5.4, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, Glucose 10; carbogen bubbled to pH 7.4), cardioplegic KH ([K+] raised to 20 mM), or subjected to contracture (KH equivalent where Na+ was replaced by Li+); all at 37°C. Up to three impacts were performed on each heart, and their position was documented by still and video photography.Tissue integrity was judged on site by photometric creatine kinase (CK) assay (340 nm absorbance, assay resolution 4.18 IU/L),and verified in subsequent histological studies [4]. For CK assays, coronary outflow was collected before impact (control), 3-4 times during the first minute after impact, and then at 2, 3, 5, 10, 20, and 30 min. Contrary to prior work [2], we found that samples should not be frozen but stored at 4°C, as this preserves >98% enzymatic activity for up to 24 h, whereas immersion in liquid nitrogen instantaneously reduces CK activity to <30% of the pre-freezing value and exposure to dry ice causes a reduction to <50%.Maximum pre-impact kinetic energies that did not induce tissue damage, identifiable by either CK assay or light microscopy, were 2.0-2.5 mJ for cardioplegically arrested hearts, and 5-10 mJ for contractured preparations. Tissue damage correlated well with the deceleration pathway length, which was shorter in contractured hearts. Since force (and, hence, peak pressure under the probe) is inversely related to deceleration pathway length, this suggests that the critical parameter in introducing tissue damage may, in the given experimental conditions, not be the compression of the tissue under the probe, but distension of neighbouring tissue.In the context of commotio cordis, the heart is particularly prone to developing fatal arrhythmias if impacts occur during the early T-wave. This coincides with ventricular contraction, which, according to our findings, would extend the relevant (sub-contusional) pre-impact energy range. Subsequent investigations will assess whether pre-impact speed is an independent determinant of tissue damage, and then move on to electrophysiologically active beating heart preparations.
University of Glasgow (2004) J Physiol 557P, D2
Demonstrations: Determination of mechanical energy thresholds of commotio vs. contusio cordis in Guinea pig isolated heart
I.A. MacLeod (a),P.J.Cooper (a), S.T. Schaaf (a),A. Epstein (b),C.Boulin (b) and P. Kohl (a)
(a) University Lab of Physiology, Oxford, UK and (b)EMBL, Heidelberg, Germany
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.