BACKGROUND: Respiratory syncytial virus (RSV) is a major cause of bronchiolitis and pneumonia in young infants, with an estimated 33 million cases occurring globally each year (1). Despite the high prevalence of RSV, there are currently no licensed vaccines or effective anti-viral treatments available and so supportive care remains the mainstay of therapy. A distinguishing feature of RSV bronchiolitis is the recruitment of an inflammatory infiltrate known as neutrophils into the airways of infants (2). Neutrophil-mediated factors such as neutrophil elastase (NE) and interleukin 8 (IL-8) in the blood and airways of infants hospitalised with RSV infection are thought to corelate with disease severity, although we currently do not know why (3,4). Clinical studies have also shown that neutrophils found in the systemic circulation of infants with RSV bronchiolitis contain RSV mRNA (5).
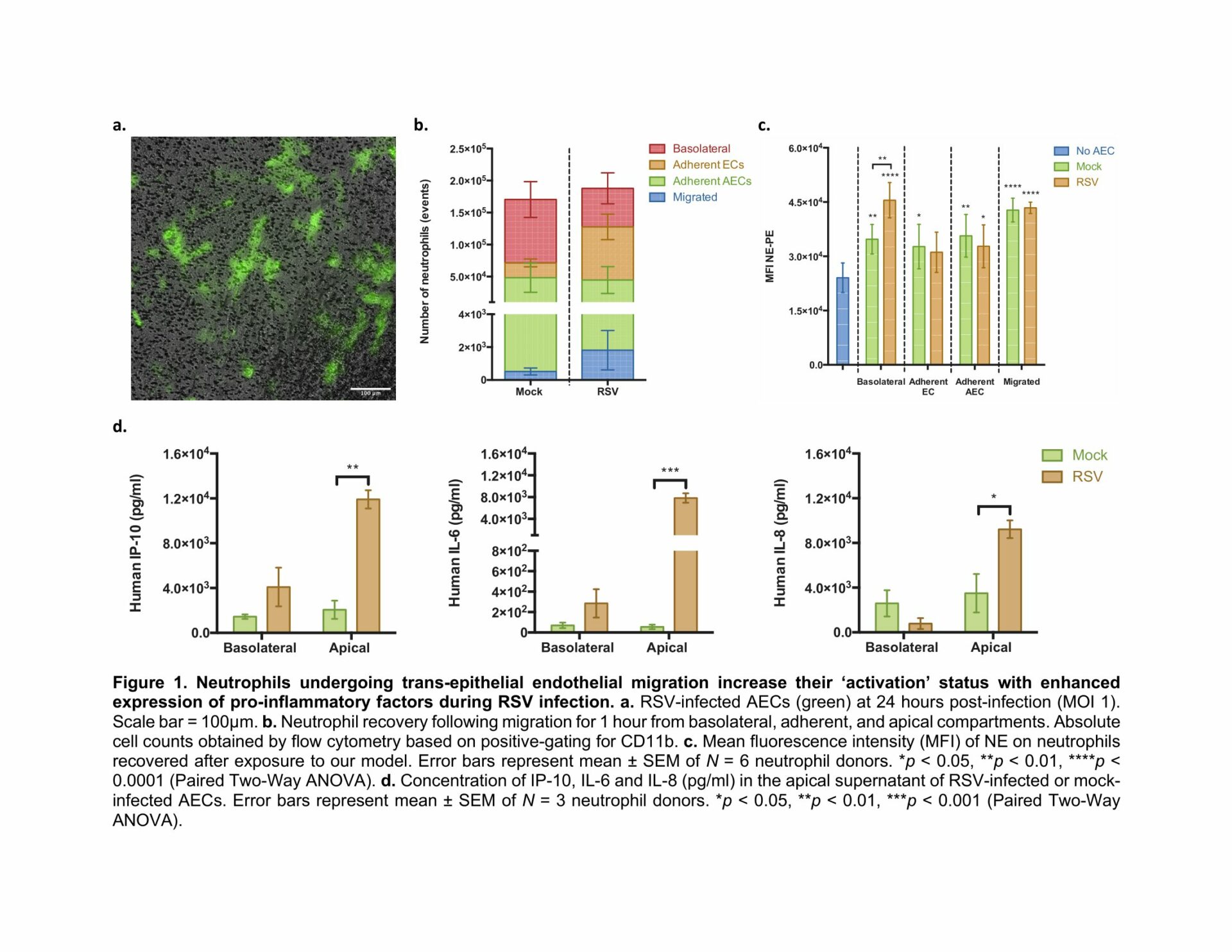
METHODS: Our lab has developed a novel trans-epithelial endothelial model of the human air-blood barrier to study neutrophil behaviour and function during RSV infection using animal-free components. To do this, primary paediatric airway epithelial cells (AECs) were grown at air-liquid interface (ALI) and co-cultured with human endothelial cells (ECs) before being infected with RSV expressing green fluorescent protein (GFP) (Figure 1A). After 24h, human neutrophils, obtained from a healthy donor, were added to the basolateral side. After 1h different sub-populations including basolateral, adherent and apical (migrated) neutrophils were recovered for subsequent analyses by flow cytometry.
RESULTS: Our data show that RSV infection led to a shift in the number of basolateral neutrophils to apical neutrophils, indicating movement across the EC/AEC barrier (Figure 1B). Exposure to RSV infected AECs led to increased expression of NE on basolateral neutrophils compared to the mock-infected control (N=6 neutrophil donors, Paired Two-Way ANOVA) (Figure 1C). This was accompanied by an increase in levels of pro-inflammatory chemokine and cytokines including interferon γ-induced protein 10 (IP-10), interleukin 6 (IL-6) and IL-8 in the apical supernatant of RSV-infected AECs compared to mock-infected control cells (Figure 1D) (N=3 neutrophil donors, Paired Two-Way ANOVA).
CONCLUSIONS: We have shown that neutrophils present on the basolateral (blood) side of RSV infected AECs increase expression of NE, which is similar to the finding of increased NE in the blood of infants hospitalised with RSV infection. These findings demonstrate that our in vitro model can replicate key human disease outcomes and can therefore be used to identify critical mechanisms that mediate epithelial cell damage and promote inflammation in children with severe RSV disease. Future work will investigate the mechanism behind this and compare the phenotype of neutrophils isolated from our model to those from the blood of RSV-infected infants, thereby allowing us to characterise a neutrophil sub-population that can be used as a biomarker of severe infection.