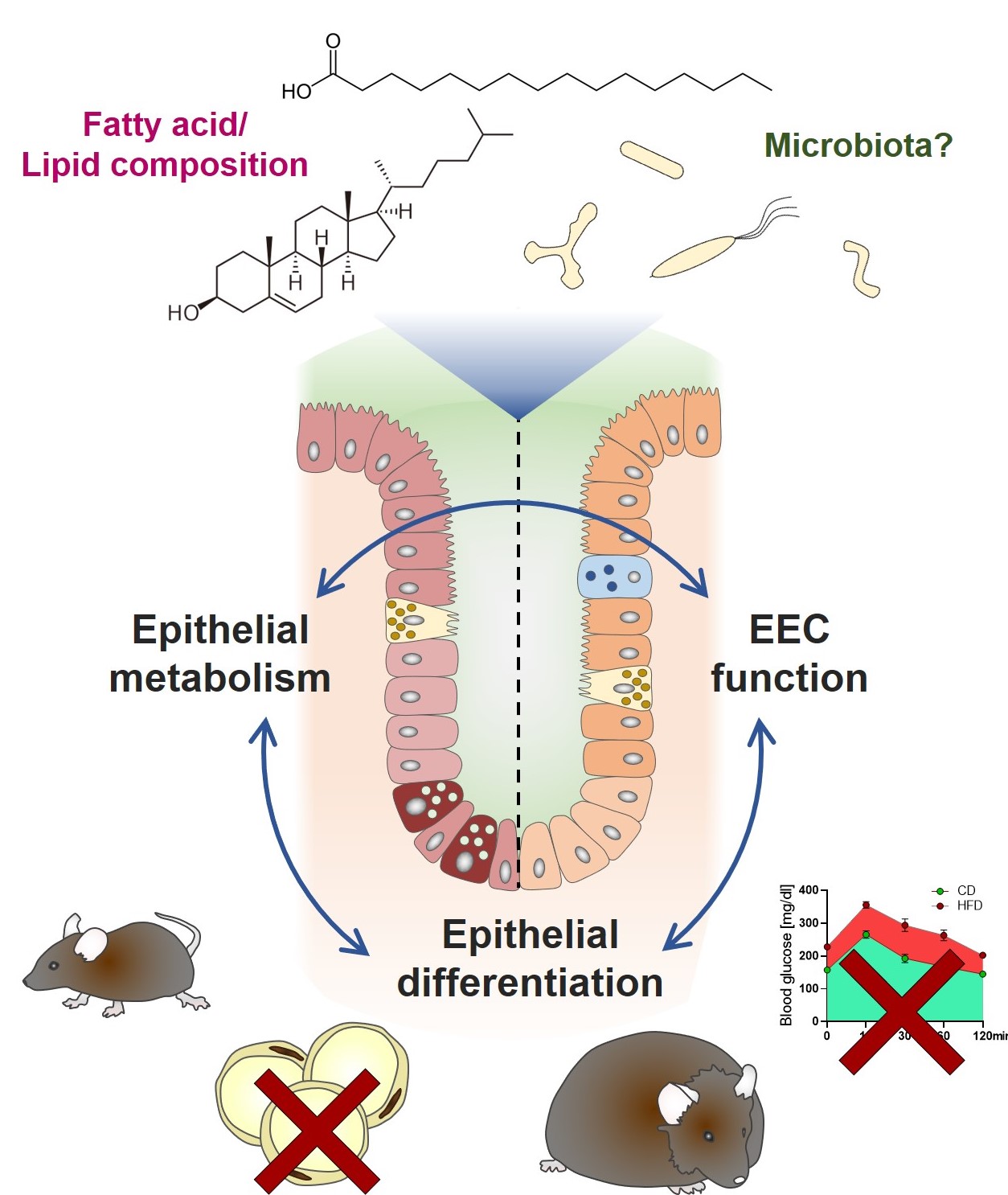
Enteroendocrine cells (EECs), produce various hormones to coordinate optimal absorptive conditions following food intake, ensuring efficient postprandial assimilation of nutrients. EEC are equipped with sensors for the detection of luminal nutrients including free fatty acids, short-chain and long-chain fatty acids. Dietary habits, obesity and microbiome composition are associated with alterations in EEC function and numbers. In particular, glucagon-like peptide 1 (GLP-1)-producing L-cells seem to be affected. Ileal versus colonic GLP-1 is suggested to confer location-specific functions, stimulating insulin release and reducing gastrointestinal motility, respectively.
To scrutinize the effect of dietary fat and obesity on L-cell density in the intestinal epithelium, we quantified GLP-1+ cells in the ileum and colon of mice. BL/6J mice were fed high-fat diets (HFD) based on different fat sources (palm oil (P), lard (L)) (n= 6 – 8 in all feeding groups). Effects of the P-HFD were characterized over time (1, 4, 12 weeks) and for 48 and 60 kJ% of fat. Additionally, using mouse strains with different susceptibilities to diet-induced obesity (DIO), AKR/J (high), BL/6J mice (intermediate), and SWR/J (low/none), we investigated P-HFD 48-mediated effects independent of obesity. L cells per open crypt were quantified in at least 3 non-consecutive tissue sections based on immunohistochemical stainings for Glp-1. All samples analyzed in this study for EEC/ EC numbers were obtained from our tissue biobank and generated in the context of a previously published study (doi: 10.1002/mnfr.201400840). Thus, in accordance with 3R principles, no mice were sacrificed for the purpose of this study. In accordance with previous reports indicating enhanced L-cell differentiation induced by dietary lipids, P-HFD increased GLP-1+ cell numbers in the ileum and colon of BL/6J mice. In the colon, the effect was already significant after one week (1.98 fold increase (x) over control diet, p< 0.0001, t-test), was enhanced after 4 weeks (2.54x, p< 0.0001) but stayed stable afterwards (2.43x, p< 0.0001), without additional impact of fat content (48 versus 60 kJ%). In contrast, 4 weeks on L-HFD caused only a borderline significant increase in ileal GLP-1+ cells (1.27x, p< 0.0149), but no significant changes in the colon. Corroborating the hypothesis that dietary fat directly impacts EEC differentiation and not obesity per se, the increase in colonic GLP-1+ cells was similar in all mouse strains, independent of weight gain. In line with a role of colonic GLP-1 in controlling intestinal transit time, numbers of GLP-1+ cells did not correlate with basal blood glucose levels or AUC in SWR/J mice (Spearman correlation). The main difference in composition between P-HFD and L-HFD is the content of palmitic acid and cholesterol (exclusively present in L-HFD). GLP-1+ cell numbers remained unaltered in L-HFD fed mice, thus palmitic acid or its metabolites might directly foster EEC differentiation. In contrast, the effect of HFDs on metabolic parameters does not seem to affect intestinal L cell numbers.
This study highlights potential of precise nutritional interventions in the context of metabolic diseases and underlines the necessity for careful interpretation of data from DIO models due to distinct effects of the fat source.