Expression of the regulator of G-protein signalling (RGS) proteins results in rapid deactivation of G-protein activated inwardly rectifying K+ (GIRK) channels, consistent with their catalytic function as a GTPase-activating protein on active Gα subunits and their role as negative regulators of other G-protein pathways. Unexpectedly RGS proteins also accelerate receptor-stimulated activation kinetics of GIRK channels, by an unknown mechanism. The effects of neuronal RGS8 were examined in HEK-293 cell lines, stably expressing GIRK channel subunits (Kir3.1+3.2A) plus one of the following GPCRs: A1 adenosine, α2A adrenergic, D2 dopamine, M4 muscarinic, or the GABA-B1b/2 heterodimeric receptor.
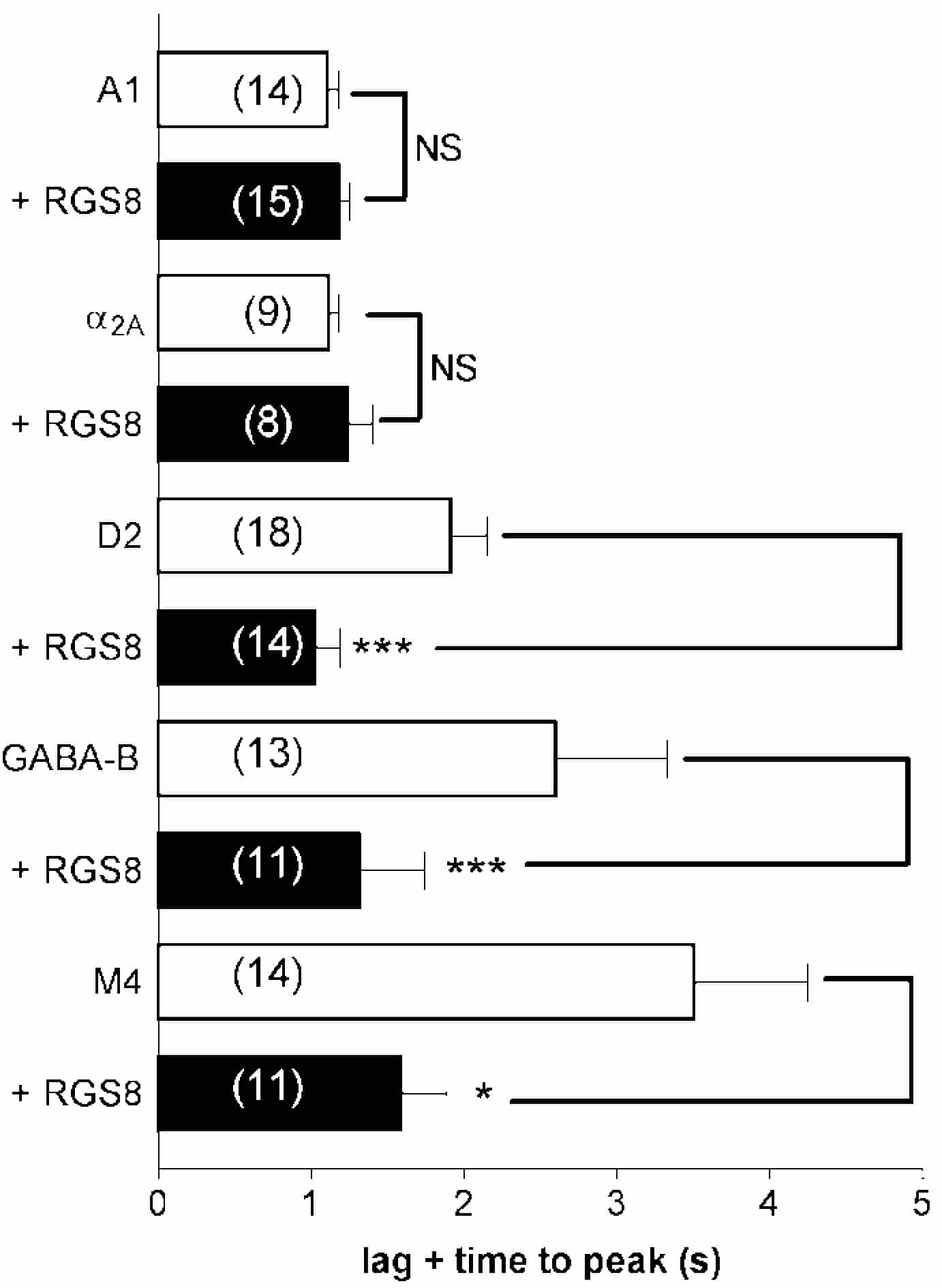
Whole-cell K+ currents in symmetrical K+ (140 mM) were recorded at a membrane potential of -60 mV. Currents were activated by fast application and removal of agonists (delay time approx. 0.25s): GABAB, 100 µM baclofen; M4, 100 µM carbachol; D2, 10 µM dopamine; A1, 1 µM adenosine; α2A, 3 µM noradrenaline. The kinetics of receptor-stimulated currents were characterised by measuring the lag and time to peak current amplitude (lag+TTP) for activation, and by fitting a single exponential time constant (t{special}deact) to the deactivation phase. Data are given as means ± S.E.M. and were reciprocated prior to statistical analysis with Student’s unpaired t test.
The main findings were that transfection of RGS8 (tagged with the yellow fluorescent protein, RGS8-YFP) significantly accelerated current deactivation in each of the receptor cell lines (compared to control recordings) but a receptor-selective effect was seen on activation kinetics whereby activation of currents via the D2, GABAB and M4 receptors was significantly accelerated whilst activation via A1 and α2A receptors was not affected by RGS8-YFP expression (GABAB, lag+TTP(s): 2.6 ± 0.7, n = 13; + RGS8: 1.3 ± 0.4, n = 11, P < 0.001; A1, lag+TTP: 1.1 ± 0.01, n = 14; +RGS8: 1.2 ± 0.01, n = 15, NS). The lag TTP data for all receptors studied is shown in Fig. 1. These effects were dependent on the RGS catalytic domain since an N-terminally deleted construct exhibited identical kinetic effects to RGS8-YFP. The receptor selectivity of RGS8-YFP was not due to preferential regulation of certain Gα-isoforms because, when signalling was constrained to transfected PTx-insensitive GoαA(CŒ{special}G) subunits in the GABAB and A1 cell lines (treated with PTx at 100 ng ml-1 for >16 h), RGS8-YFP accelerated GABAB-mediated activation (lag+TTP(s): +GoαA: 3.3 ± 0.6; +GoαA + RGS8: 1.2 ± 0.1, P < 0.001; n = 8) but not A1-mediated activation of currents (lag+TTP: +GoαA: 2.2 ± 0.3; +GoαA + RGS8: 2.5 ± 0.3; n = 6, NS). Deactivation was significantly accelerated in both lines. Our data support current ideas of kinetic scaffolding and indicate that this occurs in a receptor-dependent fashion.
This work was funded by the Wellcome Trust and BHF.