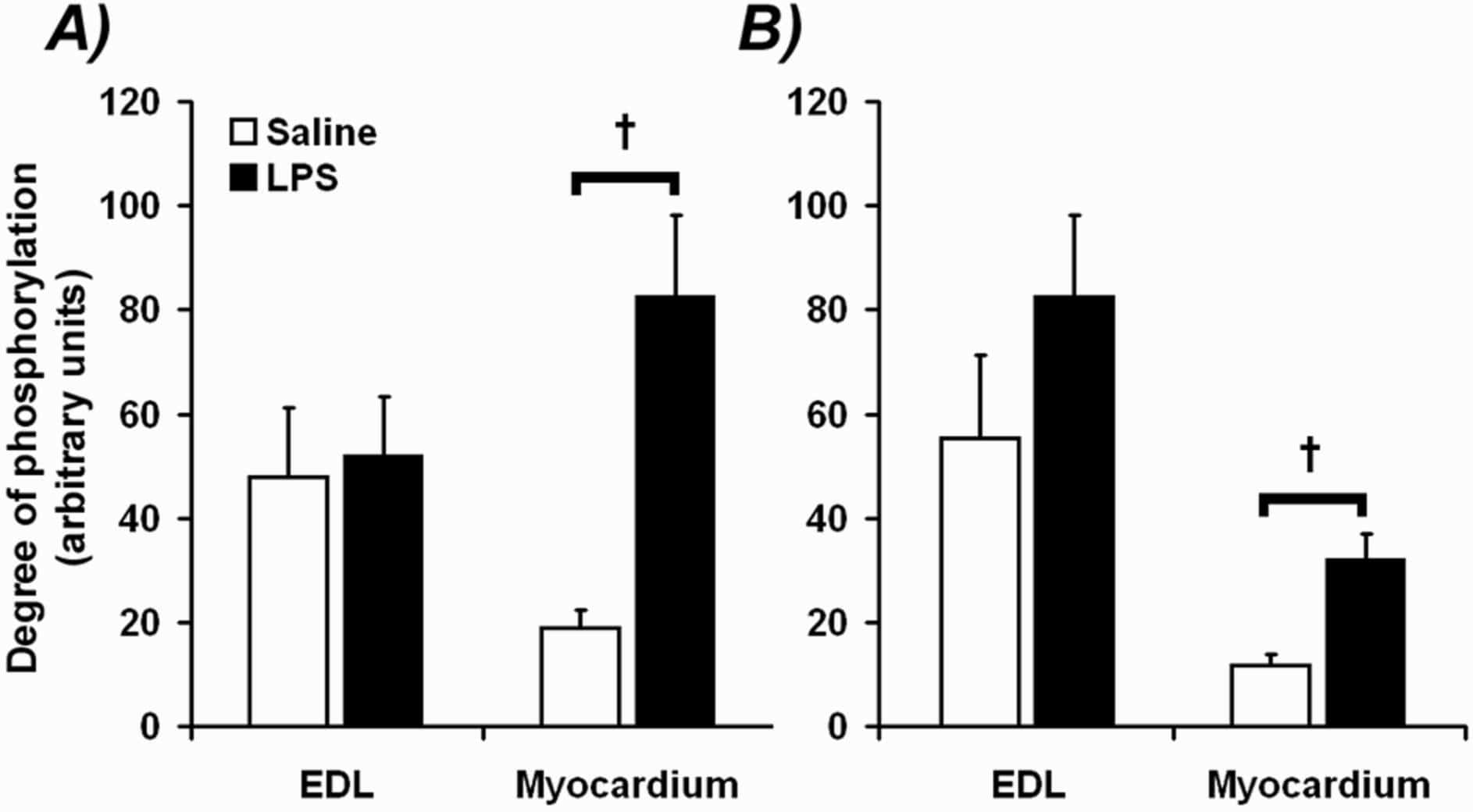
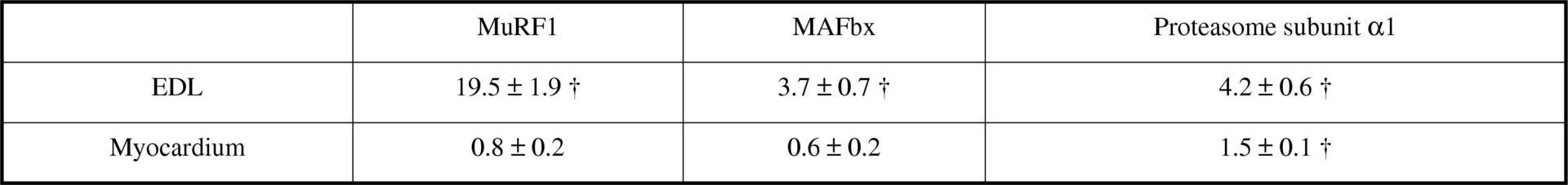
It is unclear whether during endotoxaemia, the myocardium is protected from the same rapid reduction in protein content that is classically observed in fast-twitch skeletal muscle (Crossland et al., 2008) and if so, whether this is reflected by differential responses in the molecular mechanisms thought to regulate tissue protein content. To investigate this further, conscious male Sprague Dawley rats, previously implanted with jugular venous catheters under general anaesthesia (fentanyl and medetomidine, 300 μg kg-1 each i.p.), were administered lipopolysaccharide intravenously for 24 h (LPS; 150 μg kg-1 h-1; n=6) or, as means of a control, saline (0.4 ml h-1; n=8), after which the extensor digitorum longus (EDL) and myocardium were removed under terminal anaesthesia (sodium pentobarbital, i.v.). The protein-to-DNA ratio, a marker of protein content, was significantly reduced in the EDL following LPS infusion (23% ± 17% (SE); P<0.05), but was no different from controls in the myocardium. Furthermore, a significant increase in components associated with ubiquitin-proteasome mediated protein degradation, namely MAFbx, MuRF1 and 20S proteasome subunit α1 mRNA levels, was observed in the EDL compared to control, but not in the myocardium (Table 1). In contrast, an elevation in phosphorylation of p70 S6K Thr421/Ser424 residues (P<0.05), and 4E-BP1 Thr37/Thr46 residues (P<0.05), was seen exclusively in the myocardium of LPS-treated animals (Fig. 1), consistent with an elevation in protein translation initiation. In summary, these findings suggest that the myocardium does not undergo the same atrophy response as skeletal muscle during endotoxaemia, at least partly due to the absence of genomic and signalling events in the myocardium typically associated with increased muscle proteolysis and the suppression of protein synthesis.
King's College London (2009) Proc Physiol Soc 14, PC14
Poster Communications: Differential effects of endotoxaemia on protein metabolism in fast-twitch skeletal muscle and myocardium of the rat
A. J. Murton1, N. Alamdari1, S. M. Gardiner1, D. Constantin-Teodosiu1, R. Layfield1, T. Bennett1, P. L. Greenhaff1
1. Centre for Integrated Systems Biology & Medicine, School of Biomedical Sciences, The University of Nottingham, Nottingham, United Kingdom.
View other abstracts by:
FIGURE 1: A) p70 S6K Thr421/Ser424 and B) 4E-BP1 Thr37/Thr46 phosphorylation in EDL and myocardium in response to LPS infusion. Values are mean ± SE (arbitrary units). † significantly different from control (P<0.05 ANOVA).
TABLE 1: Fold changes in MuRF1 MAFbx and proteasome subunit &#945;1 mRNA levels from corresponding control value in EDL and myocardium in response to LPS.<#13>Values are mean &#177; SE. &#8224; Indicates different from control (P&lt;0.05 ANOVA)
Where applicable, experiments conform with Society ethical requirements.