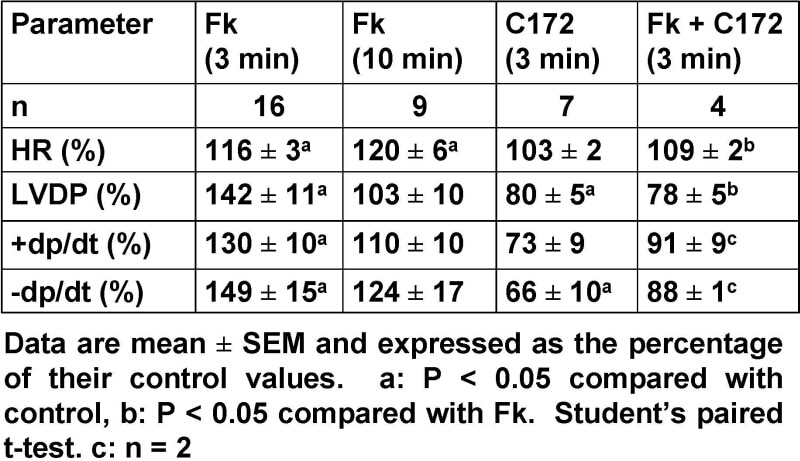
The cystic fibrosis transmembrane conductance regulator (CFTR) Cl– channel is expressed in the mammalian heart (Hume et al. 2000). However, its cardiac function is poorly understood in part due to lack of specific tools to manipulate CFTR activity. Recently, Ma et al. (2002) demonstrated that the thiazolidinone CFTRinh-172 (C172) is a potent, specific inhibitor of the CFTR Cl– channel. To investigate the cardiac function of CFTR, we tested the effects of C172 on isolated perfused rat hearts. Male Wistar rats (250-275 g) were killed by cervical dislocation. The hearts were removed and mounted on a Langendorff apparatus and perfused with Krebs solution (37 °C) at a constant flow rate of 9 ml min-1. A balloon was inserted into the left ventricle to monitor ventricular pressure, heart rate and other haemodynamic parameters using a data acquisition system (Chart v4.0.2, PowerLab ADInstruments). Data were analysed offline. To stimulate CFTR by increasing intracellular cAMP levels, we used forskolin (Fk, 0.5 μM). To inhibit CFTR, we used C172 (10 μM, Asinex Ltd), a concentration that inhibits maximally CFTR-mediated Cl– secretion in epithelia (Ma et al. 2002). Drugs were dissolved in Krebs solution. Individual rat hearts were treated randomly with either Fk, C172 or Fk + C172 with C172 added 2 min after Fk. Our results are summarised in the Table. Fk (0.5 μM) induced an immediate change in all the parameters within the first 3 min of perfusion. However, at the end of a 10 min perfusion period, HR continued to increase, while other parameters returned to their control levels. In contrast, over a 3 min perfusion period, C172 (10 μM) induced an immediate reduction in LVDP and −dp/dt. However, HR and +dp/dt were not significantly affected by C172. On washing C172 away, we observed a transient increase in LVDP. When administrated to Fk pretreated hearts, C172 abolished the immediate positive inotropic and chronotropic effects of Fk. Vehicle (0.1% DMSO) was without effect (n = 4, data not shown). Our data suggest that the immediate effects of either the CFTR blocker C172 or the CFTR activator Fk are not consistent with the predicted negative inotropic role of CFTR in the heart (Hume et al. 2000). We speculate that the immediate effects of Fk might be due to other mechanisms such as the activation of the L-type Ca2+ channel. Further studies are required to determine whether C172 affects cardiac function by CFTR-dependent or independent mechanisms.
King's College London (2005) J Physiol 565P, PC92
Communications: Direct effects of the specific CFTR blocker CFTRinh-172 on the function of isolated rat heart
Li, Hongyu ; Lin, Huan ; Sheppard, David N.; Suleiman, M.-Saadeh ;
1. Physiology, The University of Bristol, Bristol, United Kingdom. 2. Bristol Heart Institute, Bristol Royal Infirmary, Bristol, United Kingdom.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.