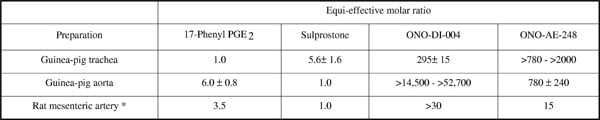
Discrimination of EP1 and EP3 prostanoid receptors has mainly relied on the use of selective agonists in combination with EP1 antagonists, although EP3 antagonists are now emerging. We were concerned about the lack of functional data for two newer agonists, ONO-DI-004 (EP1-selective) and ONO-AE-248 (EP3-selective) (Suzawa et al., 2000). Consequently, we have examined these agents on guinea-pig trachea (EP1 preparation) and guinea-pig aorta and rat mesenteric artery (EP3 preparations). Ring preparations obtained from male guinea-pigs (600 – 800 g) and male rats (250 – 300 g) were suspended in Krebs-Henseleit solution (95% O2 / 5% CO2) at 37oC in 10 ml tissue baths. Tension was recorded with a force transducer and relayed to AD Instruments Chart software. Cumulative agonist sequences were obtained in the presence of the COX inhibitor indomethacin (1 µM) and the TP antagonist BMS-180291 (300 nM), and under priming with 20 mM K+ on guinea-pig aorta. The agonist potencies of 17-phenyl PGE2 (EP1 > EP3), sulprostone (EP3 > EP1), ONO-DI-004 and ONO-AE-248 are shown in Table 1. EC50 values for 17-phenyl PGE2 on trachea were 0.74 – 1.3 – 2.0 nM (mean / range) and for sulprostone on aorta and mesenteric artery were 0.079 – 0.29 – 0.87 nM and 7 – 11 – 16 nM respectively. ONO-AE-248 had a shallower log concentration-response curve than sulprostone on guinea-pig aorta. The EP1 antagonists SC-51322 (0.03 – 1 µM) and AH-6809 (0.1 – 3 µM) abolished established responses to 10 nM 17-phenyl PGE2 / 1.4 µM ONO-DI-004 on guinea-pig trachea; pA2 values computed by the Gaddum method were 8.09 ± 0.05 / 7.99 ± 0.10 and 7.61 ± 0.04 / 7.48 ± 0.02 (± SEM, n = 4) respectively. Comparable studies are in progress with an EP3 antagonist. It is clear that ONO-DI-004 and ONO-AE-248 show high selectivity for EP1 and EP3 receptors respectively. However, this occurs at the expense of considerable loss of potency, to the extent that it would be difficult to use these agonists in a Schild antagonism protocol. They could be used in a Cheng-Prusoff protocol (Leff & Dougall, 1993), with the advantage that their onset and offset of action are faster than those of 17-phenyl PGE2 and sulprostone, which probably reflects their lower potencies / affinities.
Life Sciences 2007 (2007) Proc Life Sciences, PC358
Poster Communications: Discrimination of EP1 and EP3 prostanoid receptor systems in smooth muscle using ONO-DI-004 and ONO-AE-324
R. L. Jones1, W. A. Wan Ahmad1, R. M. Wadsworth1, D. F. Woodward2
1. Strathclyde Institute of Pharmacy & Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom. 2. Department of Biological Sciences, Allergan, Irvine, CA, USA.
View other abstracts by:
Table I. Relative potencies of EP receptor agonists
Where applicable, experiments conform with Society ethical requirements.