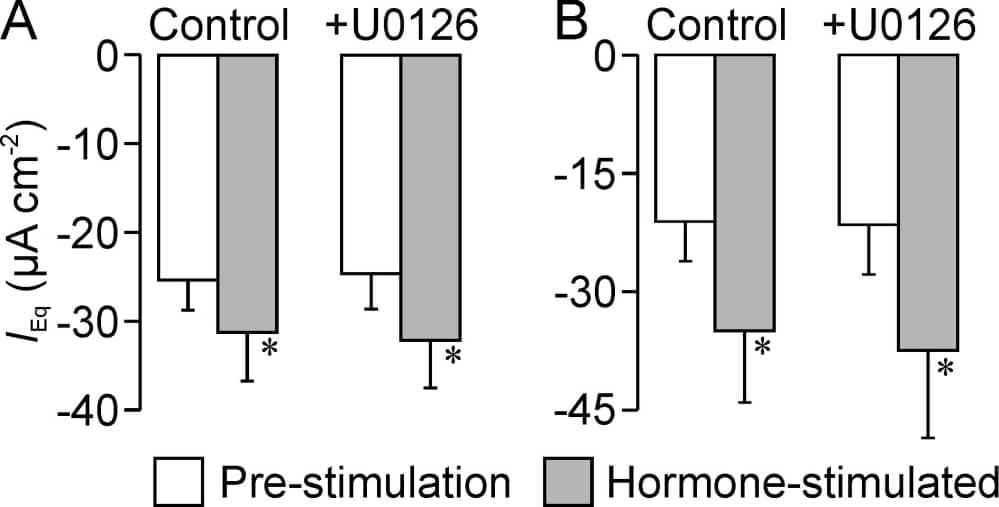
ENaC-mediated absorption of Na+ in the distal nephron is crucial for whole body Na+ and water balance and therefore important to the control of blood pressure (Loffing and Korbacher, 2009). ENaC is regulated via complex mechanisms and studies of Xenopus oocytes expressing α-, β- and γ-ENaC indicate that phosphorylation of β- and γ-ENaC by ERK1/2 may restrict membrane Na conductance by facilitating the internalisation / degradation of ENaC subunits. ERK1/2 activity may thus contribute to the hormonal control of Na absorption (Yang et al., 2006), and the present study therefore explores the effects of an inhibitor of ERK1/2 (U0126) upon electrogenic Na+ transport in a mouse cortical collecting duct cell line (mpkCCD). Cells grown to confluence on permeable membranes were therefore mounted in Ussing chambers where electrogenic Na+ transport was quantified using standard electrometric methods (see for example Manley & Wilson, 2010). Western analysis of extracted protein (n = 4) showed that U0126 (10 mM, 30 min) suppressed the phosphorylation of ERK1/2 at Tyr202 / Tyr202 indicating full inhibition of this kinase. Although U0126 (10 mM) also caused slight (5 – 10%) inhibition of amiloride-sensitive equivalent current (IEq), this effect was transient and, after 30 min, the electrical properties of control and U0126-treated cells were indistinguishable (Fig. 1). Basolateral insulin (20 nM, Fig 1A) and arginine vasopressin (AVP, 10 nM, Fig 1B) consistently augmented IEq and subsequent addition of apical amiloride (10 µM) caused ~95% inhibition of the current recorded from hormone-treated cells indicating that these responses are due to increased Na+ absorption via ENaC. Western analysis (n = 4) showed that insulin (20 nM, 30 min) also stimulated the phosphorylation of a endogenous residue (PKB-Ser473) that is phosphorylated in a phosphoinositide-3-kinase (PI3K) -dependent manner but had no effect upon the phosphorylation of CREB-Ser133, an endogenous protein kinase A (PKA) substrate. AVP, on the other hand, evoked phosphorylation of CREB-Ser133 but not PKB-Ser473 (n = 4) and these hormones thus control ENaC activity via separate mechanisms involving PI3K and PKA respectively. Studies of U0126-treated cells revealed electrometric responses to insulin and AVP essentially identical to control (Fig. 1). Despite the evidence implicating ERK1/2 in the control of ENaC activity (Yang et al., 2006), the present data show that complete inhibition of this kinase has no effect upon basal Na+ absorption and does not impair PI3K or PKA-dependent control of ENaC in mpkCCD cells.
Durham University (2010) Proc Physiol Soc 21, PC28
Poster Communications: Does the extracellular signal regulated kinase 1/2 (ERK1/2) contribute to the hormonal control of epithelial Na+ channel (ENaC) activity?
S. Simpson1, M. K. Mansley1, S. M. Wilson1
1. Centre for Cardiovascular and Lung Biology, University of Dundee, Dundee, United Kingdom.
View other abstracts by:
Figure 1. Confluent mpkCCD cells were mounted in Ussing chambers, allowed to stabilise for approximately 40 min and then exposed to 10 µM U0126 (+U0126) or solvent vehicle (control) for 30 min. The cells were then stimulated with either insulin (A, 20 nM, basolateral, n = 5) or arginine vasopressin (B, 10 nM, basolateral, n = 5). Open columns show equivalent currents (IEq) monitiored under open circuit conditions that were quantified immediately before the cells were exposed to hormones, whilst shaded columns show currents recorded after 30 min stimulation. Data are mean ± s.e.m. and asterixes denote statistically significant (P < 0.05, Student’s paired t test) effects of hormones upon IEq.
Where applicable, experiments conform with Society ethical requirements.