Rhythmical contractions of the gastrointestinal tract are associated with the pacemaking electrical activity generated in the muscle layers: it occurs at low frequency in the absence of an extrinsic nervous stimulation. Interstitial cells of Cajal (ICC), distributed in the myenteric region of the gastric wall, have been suggested to initiate the pacemaking activity (Tomita, 1981). This pacemaking activity then propagates to the smooth muscle cells through gap junctions to generate the contraction of the whole gastrointestinal tract. This pacemaking activity is recorded as the driving potential of ICC.
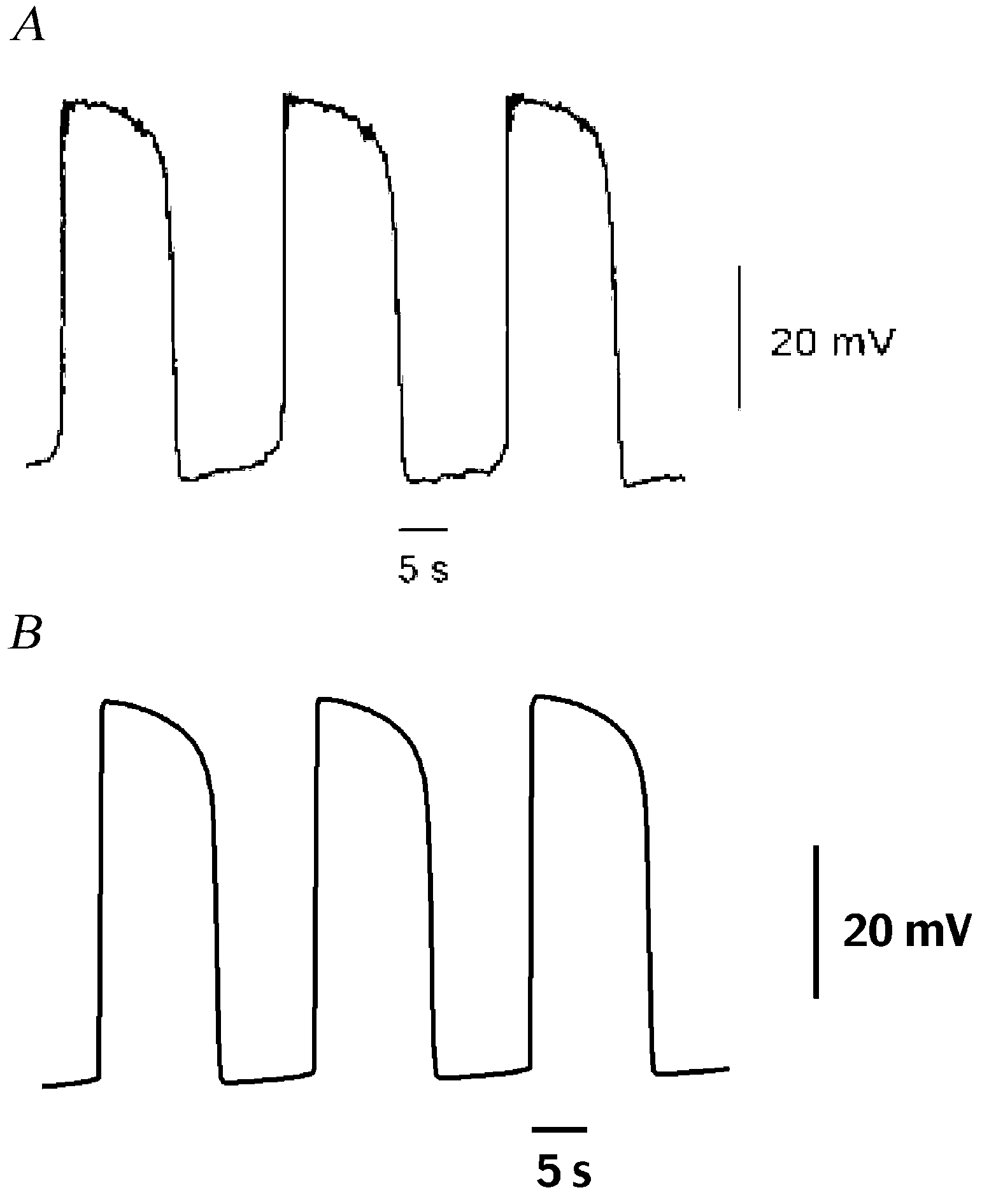
In this study, driving potentials of ICC from experimental records were simulated with a mathematical model to explain the regenerative nature of the slow potentials and the underlying [Ca2+]i changes. Included in the model are the apparatus for [Ca2+]i regulation and pacemaker current. Ca2+ entry through a dihydropyridine-resistant conductance triggers IP3-mediated Ca2+ release (Ward & Sanders, 1992). Subsequent mitochondrial Ca2+ uptake transiently reduces [Ca2+]i in the space close to the non-selective cation channels. Activation of these channels generates the pacemaker current and it makes the initial component of the driving potential (Sanders et al. 2000). The depolarization by the pacemaker current facilitates the production of IP3 and the plateau phase of the driving potential continues (Ganitkevitch & Isenberg, 1993). Gradual decrease of IP3 and [Ca2+]i terminates the plateau phase. In the model, the membrane potentials between successive driving potentials are in the range from -66 to -72 mV (Fig. 1). Peaks of driving potentials are between -30 and -25 mV. The duration of driving potentials ranges from 8 to 12 s. Analysis of behaviours of the pacemaker current and the [Ca2+]i regulation in the model reveals the complexity of the underlying process in the generation of the driving potential.
This work was supported by a research fund from Advanced Backbone IT Technology Development Project from Ministry of Information and Communication (IMT-2000-C3-5).