The action potential (AP) propagates by the concerted action of voltage-gated ion channels, whose probabilistic behaviour introduces channel noise. In thin unmyelinated axons, typical for the mammalian cortex (av o.d. 0.3 µm), channel noise jitters the timing of APs (Faisal et al. 2002), generates spontaneous APs (Faisal et al. submitted) and varies the AP wave form. The wave form of the presynaptic AP is of fundamental importance in determining the strength of synaptic transmission (e.g. Sabatini & Regehr (1997)). However, the variability of the AP wave form in thin axons is unknown, because it is difficult to record fast signals from within such small structures.
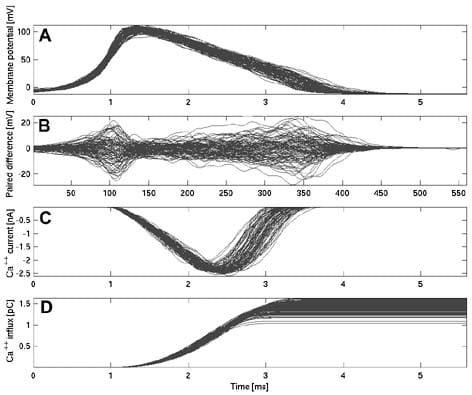
We developed a stochastic simulator and simulated a 1.6 mm long unmyelinated pyramidal cell axon collateral of 0.2 µm diameter (Na and K channel densities 60 µm-2 and 18 µm-2, channel conductance 20 pS, Ra = 70 V cm, Rm = 20000 V cm2, resting potential -65 mV), which was stimulated with white noise current (0 nA ± 0.01 nA) at its proximal end. The travelling AP wave form fluctuated considerably (Fig. 1, n = 713). AP width (at half-peak) measured mid-way down the axon and at its terminal had coefficients of variation (CV = S.D./av) of 8 % (1.6 ms ± 0.11 ms S.D.) and AP height (resting potential to peak) had CVs of 4 % (103 mV ± 4.5 mV). Individual APs showed only weak correlation in their wave forms at mid-axon and the terminal end (correl coef 0.08 and 0.05 for AP width and height). Thus, wave form variability results from channel noise and not from the stimulus. To determine how form variability affects post-synaptic responses, we compared it to data from a variety of large synapses. These show consistently a linear relationship between AP width and EPSC amplitude. A CV in AP width of 8 % would translate e.g. for a Granule Cell to Purkinje Cell synapse (Sabatini & Regehr, 1997) into a CV of 50 % for the EPSC amplitude. The AP wave form determines the calcium signal that controls vesicle fusion, by both controlling the opening of voltage-gated calcium channels and the driving force for calcium influx. Integrating our simulated AP wave forms into a model of a Calyx-of-Held type synapse (Borst & Sakmann, 1998) shows that both calcium peak current and total calcium ion influx have a CV of 9 %, which translates (Augustine, 2001) into a change in vesicle release probability of over an order of magnitude. Synaptic reliability and variability has been in general attributed to mechanisms inside the cortical synapse, but our knowledge is based on paired soma recordings. Thus, it is difficult to dissociate synaptic and axonal stochastic effects. Although care has to be taken when relating data from large synapses to much smaller cortical synapses, our simulations suggest that axonal channel noise could have an impact on cortical synaptic transmission.
AAF is a Boehringer-Ingelheim Fonds Ph.D. Fellow, a BBSRC supported research student and a honorary scholar of the Studienstiftung des deutschen Volkes.