Recently a urea transporter (fUT) was cloned from frog urinary bladder (Couriaud, 1999). This transporter shares many properties with the mammalian UT-A family but unlike the UT-A isoforms, fUT is inhibited by the mercurial pCMBS. A sequence alignment comparison of fUT with mUT-A2 and mUT-A3 highlighted a Cys residue C185, which is absent from mUT-A2 and mUT-A3. The aim of the current study was to establish if C185 is linked to the sensitivity of fUT to mercurials, and whether substituting Cys residues into the equivalent positions in mUT-A2 and mUT-A3 imparts mercurial sensitivity to these transporters.
Oocytes were isolated from humanely killed Xenopus laevis, and injected with 1 ng (0.02 ng nl-1) cRNA encoding the appropriate urea transporter, its mutant or 50 nl of H2O. Point mutations were introduced using a PCR-based protocol (Quikchange, Stratagene) and verified by sequencing (Lark Technologies). Uptake of urea into UT-expressing oocytes was performed as described previously (Fenton, 2000). Oocytes were pre-incubated in ND96 containing 0.5 mM HgCl2 for 5 min before uptake. Statistical analysis was performed using Student’s unpaired t test or one-way ANOVA coupled with Tukey’s test. Statistical significance was assumed at the 5 % level.
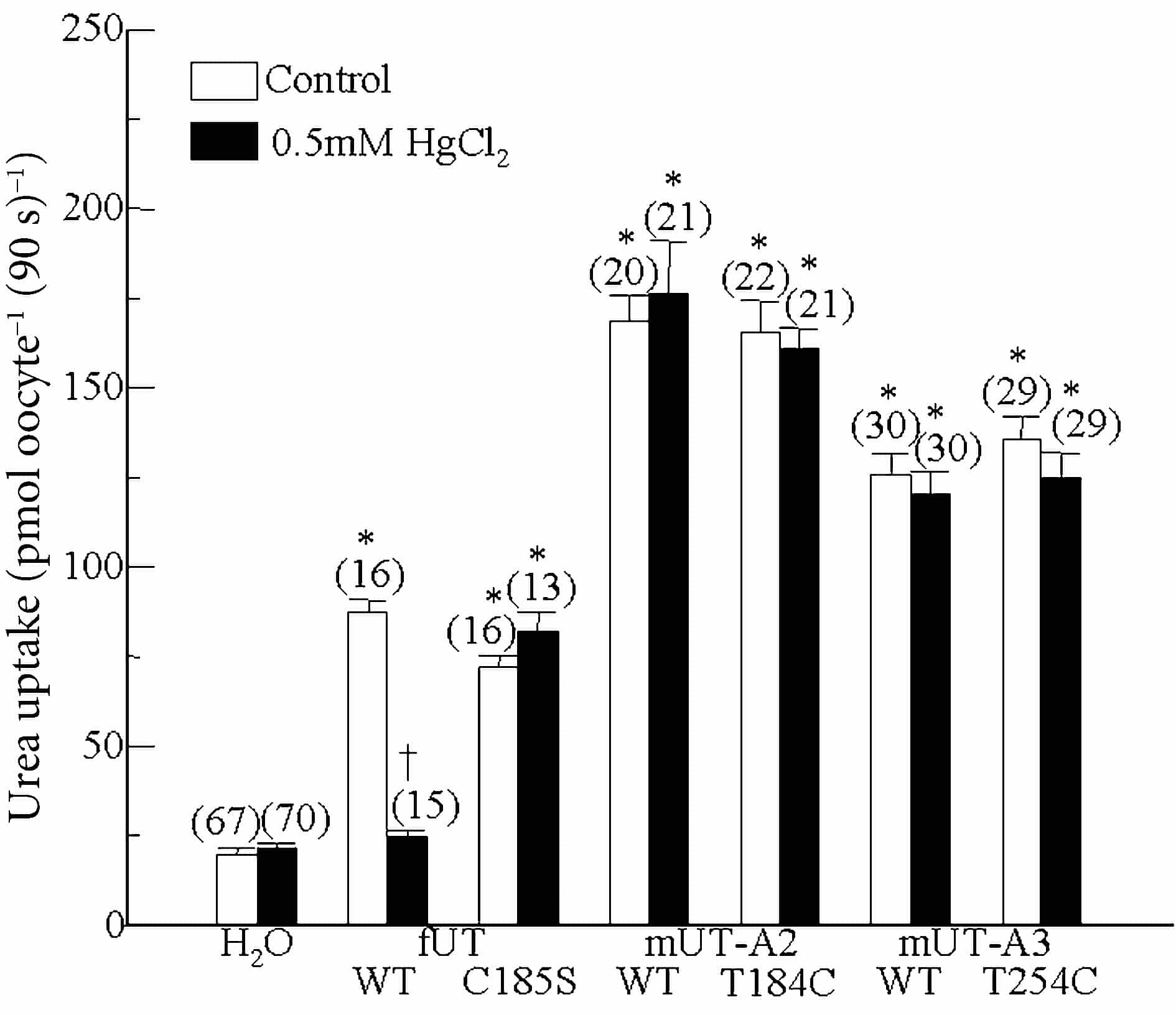
Oocytes expressing cRNAs encoding fUT, mUT-A2, mUT-A3 or their mutants exhibited a significant increase in urea uptake compared to H2O-injected oocytes (Fig. 1). In oocytes expressing fUT, Hg2+ reduced urea uptake compared to the paired control group (Fig. 1). In oocytes expressing the fUT-C185S mutant, Hg2+ no longer reduced urea uptake (Fig. 1). Cys residues were introduced into the equivalent positions in mUT-A2 (T184C) and mUT-A3 (T254C). The wild-type mUT-A2, mUT-A3 and the Cys mutants were insensitive to Hg2+ (Fig. 1).
These results indicate that C185 plays a role in the sensitivity of fUT to mercurials, implying that this residue lies near the transporter pore. The lack of effect of Cys mutations in mUT-A2 and mUT-A3 suggests either that the effect of Hg2+involves more than one residue, or that differences in the folding of mUT-A2 and mUT-A3, prevent Hg2+ binding.
The support of the Royal Society is gratefully acknowledged. fUT was a gift from G. Rousselet, CEA/Saclay, France. mT-A2 and mT-A3 were kindly provided by C. Smith (Manchester, UK).