Introduction: Sudden cardiac death remains a significant unresolved clinical problem resulting in more than 100,000 deaths per year in the UK. The majority of these deaths are due to lethal heart rhythm disturbances such as ventricular fibrillation however, the underlying mechanisms are poorly understood and there is no effective preventative treatments. Heart failure (HF) data support that the disturbance in autonomic control is a strong prognostic marker of sudden death due to ventricular arrhythmias; with sympathetic nerve activity reported to be heightened in HF. Electrical heterogeneity of the heart is also well recognised and is attributed to regional differences in sympathetic nerve innervations, ion channel distribution and action potential duration (APD); all of which become remodelled in HF. However, detailed studies into sympathetic innervations in HF and the effects on regional cardiac electrophysiology are lacking. This study aims to investigate the spatial cardiac heterogeneities in HF and the regional effects of sympathetic nerve stimulation, both across the ventricular surface and between myocardial layers, using optical mapping and voltage sensitive dyes of different wavelengths.
Methods: Adult male New Zealand White rabbits (3-3.5kg) were randomized to have coronary ligation surgeries (MI group, n=5) or sham procedures (Sham group, n=5) following premedication (Sedator 0.2mg/kg, Ketamine 10mg/kg and Torbugesic 0.05mg/kg). Anaesthesia was maintained using isoflurane and then an 8 week post-operative period was observed. Rabbits were then premedicated as before, prepared for heart isolation and administered terminal anaesthesia (Pentobarbitone sodium, 60mg i.v.). Using the isolated innervated rabbit heart preparation, optical action potentials were obtained with voltage sensitive dyes di-4-ANEPPS and di-4-ANBDQBS over the anterior left ventricle using a Hamamatsu 16×16 element photodiode array. Pacing protocols were performed via a catheter in the right ventricle, in the presence and absence of sympathetic nerve stimulation (SNS) via the spinal cord. Action potential duration (APD) and restitution (APDR) data were recorded. Data are mean±SEM; compared using ANOVA.
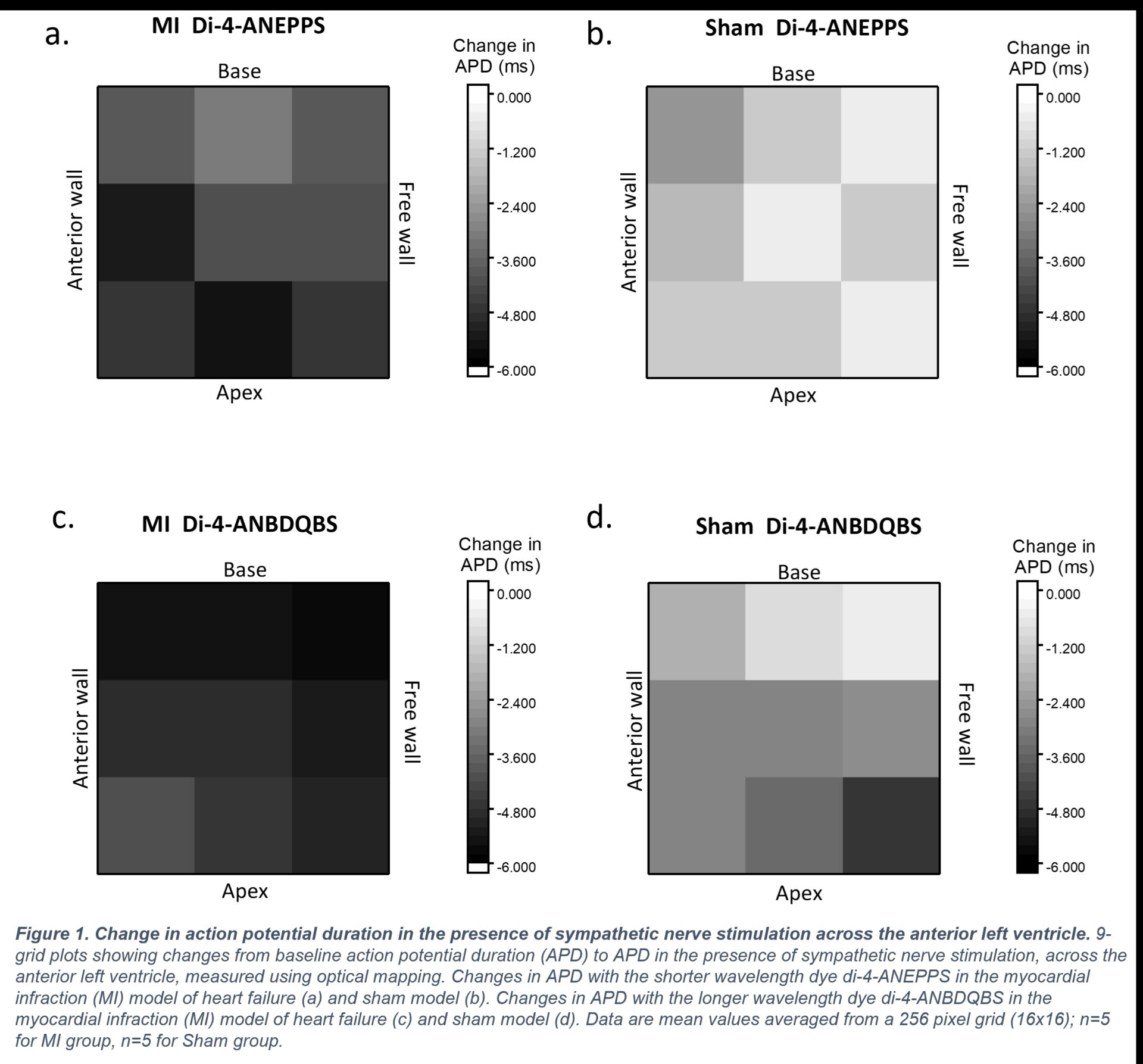
Results: MI hearts had a greater change in APD with SNS across the anterior left ventricle when compared to Shams; particularly in the mid-anterior wall (MI -5.02±2.1ms, Sham -1.25ms±0.2; p<0.05) and mid-apex (MI -5.30±2.2ms, Sham -1.24±0.2ms; p<0.05) regions when using di-4-ANEPPS. A similar response was seen with the deeper penetrating dye, di-4ANBDQBS, with the basal-freewall corner region showing the greatest change in APD in MI hearts (MI -5.56±1.3ms, Sham -3.17±0.6ms; p<0.05). These regions correlated with greater changes in in the slope of the APDR curve (di-4ANEPPS: MI mid-anterior wall 0.24±0.04, MI mid-apex 0.10±0.02; Sham mid-anterior wall 0.03±0.03, Sham mid-apex 0.06±0.03 (ns), and di-4-ANBDQBS: MI base-freewall corner 0.18±0.2, Sham base-freewall corner 0.07±0.06 (ns)).
Conclusion: MI groups showed greater, regionally specific changes in cardiac electrophysiology in the presence of SNS as a result of regionally specific heterogenous remodelling. These data detail spatial and transmural heterogeneities in electrophysiology which could be key mechanisms underlying the increased susceptibility of arrhythmia in HF. Our findings underscore the importance of regional sympathetic modulation in shaping electrophysiological responses post-MI and suggest that targeted modulation of sympathetic inputs may offer novel therapeutic potential for reducing arrhythmic risk in HF patients.