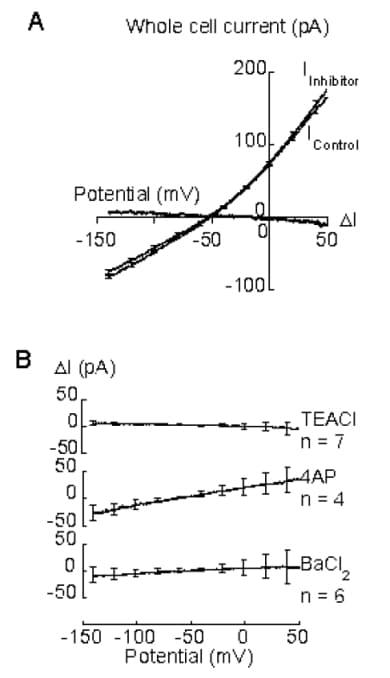
DVR are vasoactive, consisting of endothelium surrounded at intervals by pericytes, and may control distribution of renal medullary blood flow (Zhang et al. 2001, 2002). Depolarisation apparently triggers contraction of these pericytes, as of many smooth muscle cells, by opening calcium channels. These pericytes are depolarised by chloride currents (activated by angiotensin II) or by extracellular potassium (Zhang et al. 2001, 2002), but their potassium channels are unknown. Smooth muscle may express calcium-activated (KCa), voltage-dependent (Kv) and inward rectifier (KIR) potassium channels, inhibited by tetraethylammonium chloride (TEACl), 4-aminopyridine (4AP) and BaCl2,respectively (Nelson & Quayle, 1995). TEACl (30 mM) and BaCl2 (1 mM) depolarise pericytes on DVR (Zhang et al. 2002), but are nonspecific at such concentrations (Nelson & Quayle, 1995). This abstract describes the addition of TEACl (2 mM), 4AP (5 mM) and BaCl2 (50 µM) during whole cell perforated patch clamp recording of currents from pericytes on isolated DVR.Individual DVR were dissected from renal tissue kept at 4 oC (Zhang et al. 2001, 2002), after removal from rats humanely killed by stunning and cervical dislocation. DVR were incubated in collagenase and hyaluronidase (0.4 mg ml-1 of each) at room temperature for 8-9 min, stored on ice and transferred at intervals to solution at room temperature, containing (mM) Na+ 150, K+ 5, Mg2+ 1, Ca2+ 1, Cl–159, HEPES 10 and glucose 10, plus 18β-glycyrrhetinic acid (40 µM), a gap junction blocker (Turner, 2003). Heat polished pipettes containing a solution of Na+ 10, K+ 140, Cl150 and HEPES 10, plus gramicidin (0.4 mg ml-1) and dimethylsulfoxide (0.4%), were applied to pericytes to form gigaohm seals. Pericytes were clamped at -50 mV and exposed every 5 s to a ramp from -140 to +50 mV (1 mV ms-1). TEACl was added to pericytes in control solution, 4AP to pericytes in TEACl and BaCl2 to pericytes in TEACl plus 4AP. These inhibitors did not alter currents significantly (Fig 1) and therefore provide no evidence for KCa,Kv or KIR currents in unstimulated pericytes on DVR.
University of Glasgow (2004) J Physiol 557P, PC25
Communications: Effects of potassium channel inhibitors upon the electrophysiology of pericytes on descending vasa recta (DVR) isolated from rats
M.R. Turner
Swansea Clinical School, University of Wales Swansea, Swansea, UK
View other abstracts by:
Figure 1. A. Mean pericyte current (from 4 successive ramps) just before (IControl) and 1 min after (IInhibitor) addition of TEACl. Most standard error bars omitted, for clarity. I = I.B. Control - IInhibitor I calculated from currents just before and 0.5 - 3.3 min after addition of TEACl, 4AP or BaCl>2. I is not significantly different from zero (P>0.10, unpaired t test).
Where applicable, experiments conform with Society ethical requirements.