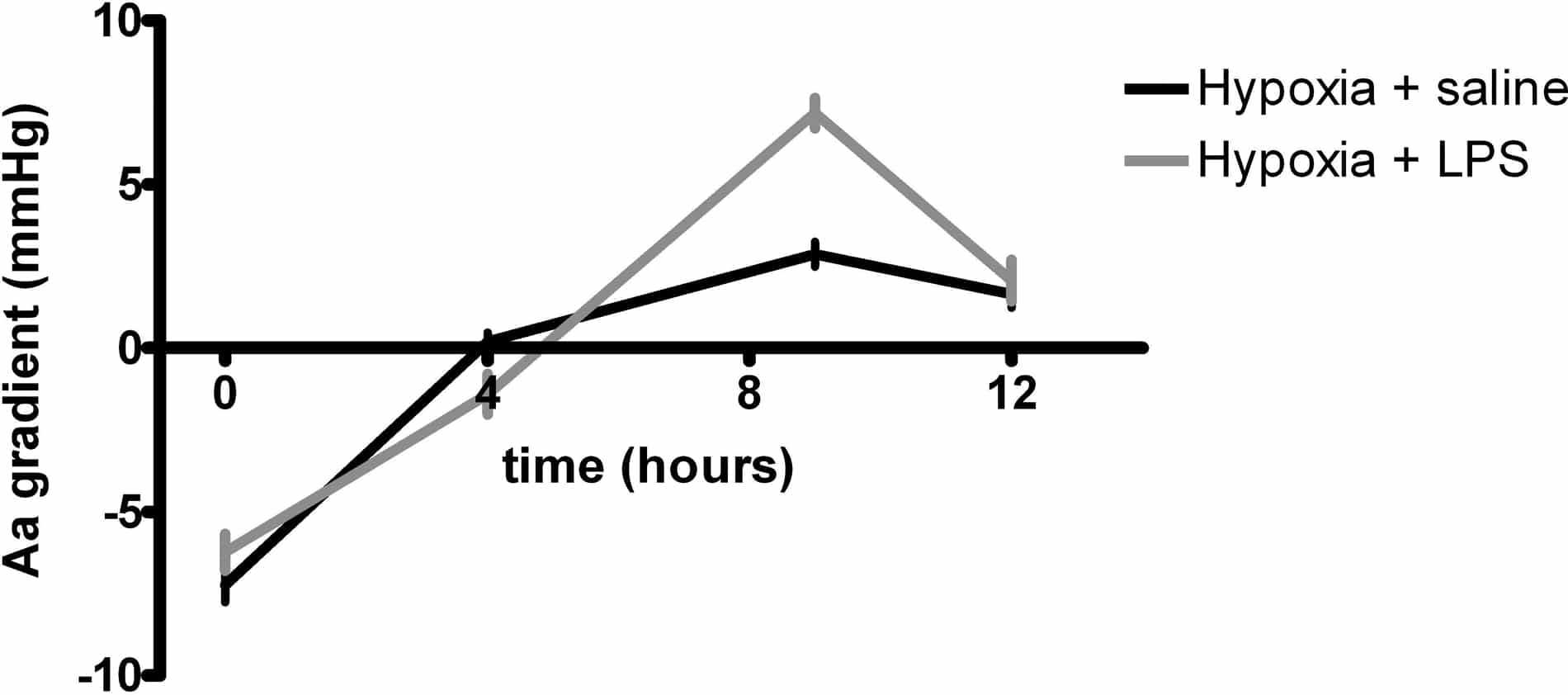
Systemic inflammation is associated with mild arterial hypoxaemia by compromising oxygen (O2) diffusion (Preas II et al, 2001) and may therefore augment the widening of the alveolar-arterial oxygen (Aa) gradient observed upon exposure to hypoxia. In the current study, we tested the hypothesis that lipopolysaccharide (LPS) infusion, a human experimental model of systemic inflammation, would further compound arterial hypoxaemia and increase the Aa gradient during acute exposure to inspiratory hypoxia in healthy humans. Twenty four healthy male volunteers aged 25 (mean) ± 4 (SD) years old were enrolled in the study after ethical approval and informed consent. In a double-blind fashion, subjects were randomised to 1) hypoxia for 12 hours (12.9% O2) and a four-hour intravenous infusion of saline from 4-8 hours, n=11 2) hypoxia for 12 hours (12.9% O2) and a four-hour intravenous infusion of LPS from 4-8 hours (total dose of 0.3 ng/kg), n =13 Arterial blood samples were obtained and the Aa gradient was calculated at 0, 4, 9 and 12 hours as FIO2(pAtm – pH2O) – (PaCO2/RQ) + (1-RQ / RQ) – PaO2, where FIO2 denotes the inspired fraction of O2, pAtm is the prevailing atmospheric pressure, pH2O is the partial pressure of water, PaCO2 is arterial PCO2, PaO2 is arterial PO2, and the respiratory quotient (RQ) is assumed to be 0.8. Inspiratory hypoxia reduced PaO2 and oxygen saturation and induced a hyperventilatory response with decreases in PaCO2 (P < 0.0001 for all, ANOVA), whereas the Aa gradient increased (P < 0.01, ANOVA). LPS infusion induced systemic neutrocytosis, increased the core temperature (P < 0.01 for both, random mixed model) and potentiated the effect of inspiratory hypoxia on the Aa gradient (P < 0.01, random mixed model; Figure 1), but affected neither the severity of arterial hypoxaemia nor the hyperventilatory response (both NS, random mixed model). In conclusion, the present findings indicate that systemic inflammation may augment the effects of acute inspiratory hypoxia on the pulmonary circulation.
University College Dublin (2009) Proc Physiol Soc 15, C83
Oral Communications: Effects of systemic inflammation on arterial hypoxaemia and the alveolar-arterial oxygenation gradient during acute inspiratory hypoxia in healthy humans
R. M. Berg1, S. Taudorf1, D. M. Bailey2, C. Lundby3, B. K. Pedersen1, K. Møller1,4
1. Centre of Inflammation and Metabolism, Department of Infectious Diseases, University Hospital Rigshospitalet, Copenhagen Ø, Denmark. 2. Neurovascular Research Laboratory, Faculty of Health and Science, University of Glamorgan, Mid-Glamorgan, South Wales, United Kingdom. 3. Copenhagen Muscle Research Centre, University of Copenhagen, Copenhagen Ø, Denmark. 4. Department of Cardiothoracic Anesthesia and Intensive Care Unit 4131, University Hospital Rigshospitalet, Copenhagen Ø, Denmark.
View other abstracts by:
Figure 1. Aa gradient during inspiratory hypoxia and LPS infusion (mean ± SEM)
Where applicable, experiments conform with Society ethical requirements.