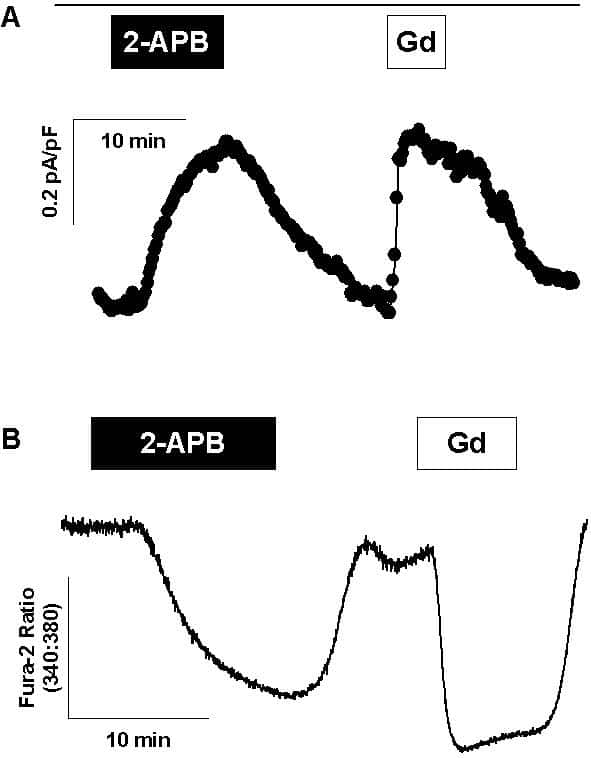
Fura-2 fluorescence measurements of cytosolic free Ca2+ concentration have led to the hypothesis that depletion of intracellular Ca2+ stores in the A7r5 rat aortic smooth muscle cell line activates a Ca2+ entry mechanism known as capacitative or store-operated Ca2+ entry (CCE or SOCE, respectively). In a recent study (1), we described a store-operated current (ISOC) in A7r5 cells, which was activated by passive depletion of Ca2+ stores with 1 μM thapsigargin or by active store depletion with 1-100 nM AVP. Because of different recording conditions, it has not been previously determined whether ISOC corresponds to CCE measured using fura-2. Nor has the channel protein(s) responsible for this Ca2+ entry pathway been identified. In the present study, the pharmacological characteristics of ISOC, including its inhibition by 2-aminoethoxydiphenylborane (2-APB), diethylstilbestrol, or micromolar Gd3+, were found to parallel the effects of these drugs on CCE measured under identical external ionic conditions using fura-2 (Fig. 1). Rat mesenteric artery smooth muscle cells (MASMC) were prepared from arteries isolated from Sprague-Dawley rats anaesthetized with isolflurane (4% by inhalation); rats were then humanely killed. Thapsigargin-stimulated ISOC in freshly isolated MASMC was similar to that recorded from A7r5 cells. Members of the TRP family of non-selective cation channels, TRPC1, TRPC4, and TRPC6 were detected by RT-PCR and Western blot in both A7r5 cells and MASMC. A stable A7r5 cell line expressing a small interfering RNA, which selectively reduced TRPC1 mRNA expression (by 43 ± 7% (mean ± S.E.M.), determined using real-time RT-PCR), exhibited significantly decreased thapsigargin-stimulated ISOC (inward current measured at -100 mV (mean ± standard error): -0.21 ± 0.02 pA/pF (n=7), compared with -0.49 ± 0.05 pA/pF (n=8) measured in control cells; p<0.001, Student's t test), suggesting that this channel contributes to the store-operated pathway.
University of Oxford (2005) J Physiol 568P, PC3
Poster Communications: Electrophysiological and pharmacological characterization of store-operated currents and capacitative Ca2+ entry in rat vascular smooth muscle cells
Brueggemann , Lioubov I.; Markun, Daniel R.; Cribbs, Leanne L.; Byron, Kenneth L.;
1. Dept. of Pharmacology, Loyola University Chicago, Maywood, IL, USA. 2. Dept. of Medicine/Cardiovascular Institute, Loyola University Chicago, Maywood, IL, USA.
View other abstracts by:
Figure 1. Similar pharmacological characteristics of ISOC and CCE A ISOC was pre-activated with 1 µ thapsigargin for 15 min. Time course of inward current recorded at -100 mV is presented. Treatment with 10 µM 2-APB for 10 min (black box) reversibly inhibits ISOC to a similar extent as 100 µM GdCl3 (white box about 5 µM free Gd3+) (representative of n=4). B recording of fura-2 fluorescence from a population of A7r5 cells pre-treated with thapsigargin to deplete intracellular Ca2+ stores and activate capacitative Ca2+ entry (CCE). 2-APB and Gd3+ have similar inhibitory effects on CCE as on ISOC (representative of 4 similar experiments). The same external solutions were used for ISOC and fura-2 recordings
Where applicable, experiments conform with Society ethical requirements.