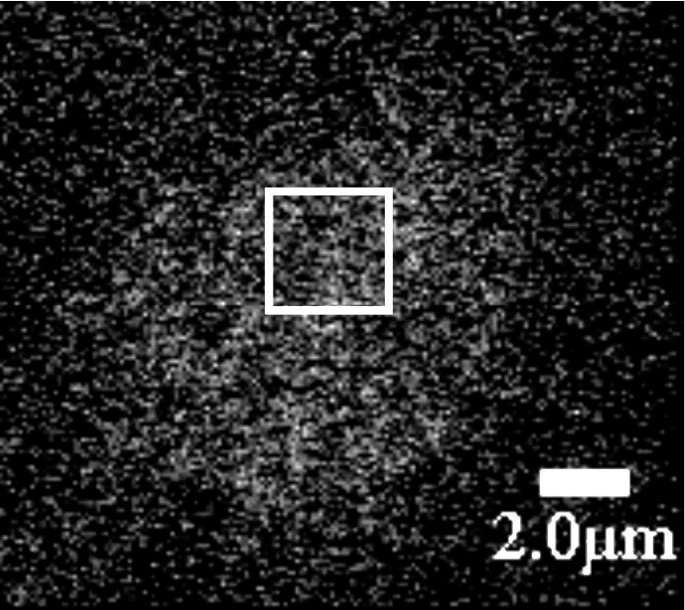
To study cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels at the cell surface we have engineered a target sequence for specific biotinylation into the fourth extracellular loop and stably expressed the mutant (CFTRbiotintag) in baby hamster kidney cells. Inserting the biotin acceptor did not compromise CFTR protein expression according to Western blots and had little effect on its channel function as assessed by iodide efflux assays and single channel recordings from excised, inside-out membrane patches. Channels were biotinylated in situ by exposing live cells to BirA, a biotin ligase that was cloned from Escherichia coli,expressed as a GST-BirA fusion protein, and added to extracellular solution containing ATP and biotin. Enzymatic biotinylation was used to purify CFTR protein on streptavidin beads. The yield of CFTR captured from cell lysates by this method was comparable to those obtained using immunopurification or Ni2+-chelate affinity chromatography of polyhistidine-tagged (10x histidine) CFTR. After incubation with BirA, subsequent exposure to a fluorescent streptavidin conjugate produced strong plasma membrane labelling of cells expressing CFTR-biotintag, but negligable staining of cells expressing wild-type CFTR or untransfected control cells. Fluorescence was not detected at the surface of cells expressing ∆F508 CFTR-biotintag, although immature protein lacking complex glycosylation was demonstrated in Western blots, consistent with defective folding and retention of this disease-associated mutant in the endoplasmic reticulum. A time series of confocal fluorescence images was used to analyze the lateral mobility of wild-type channels on the cell surface by image correlation spectroscopy (Fig. 1A). The temporal autocorrelation function r (0,0,τ) was calculated (Fig. 1B) and fitted to a two dimensional diffusion model. This yielded a diffusion coefficient of 2.0 ± 0.24 x 10-11 cmsec-1 (mean ± s.e., n = 20 cells) and immobile fraction that ranged between 0 and 43%. Similar mobilities were observed at 23 °C and 37 °C. Slow diffusion of CFTR channels in the plane of the membrane suggests their mobility may be impeded by interactions with other proteins. The results suggest that enzymatic biotinylation will be useful for purifying CFTR and studying its lateral diffusion at the cell surface.
University of Glasgow (2004) J Physiol 557P, C88
Communications: ENZYMATIC BIOTINYLATION OF WILD-TYPE AND MUTANT CFTR CHANNELS
Y. Luo (a),J.Liao (a),A.Bashir (b),D.Kolin (b),M.Wioland (a),P.W. Wiseman (b) and J.W. Hanrahan (a)
(a) Physiology, McGill University, Montreal, QC, Canada, (b) Chemistry, McGill University, Montreal, QC, Canada and (c) Physics, McGill University, Montreal, QC, Canada
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.