Background: Fetal growth restriction (FGR) resulting from placental insufficiency affects 18-30% pregnancies delivered at <32/40 weeks gestation. Compared to preterm appropriate for gestational age (AGA) human fetuses, FGR fetuses have a significantly earlier visualization of coronary artery blood flow (CABF-‘heart sparing effect’) but impaired cardiac function. Whether this dichotomy persists after birth has not been characterised. Experimental data from lambs has noted increased CABF in the setting of fetal hypoxaemia.
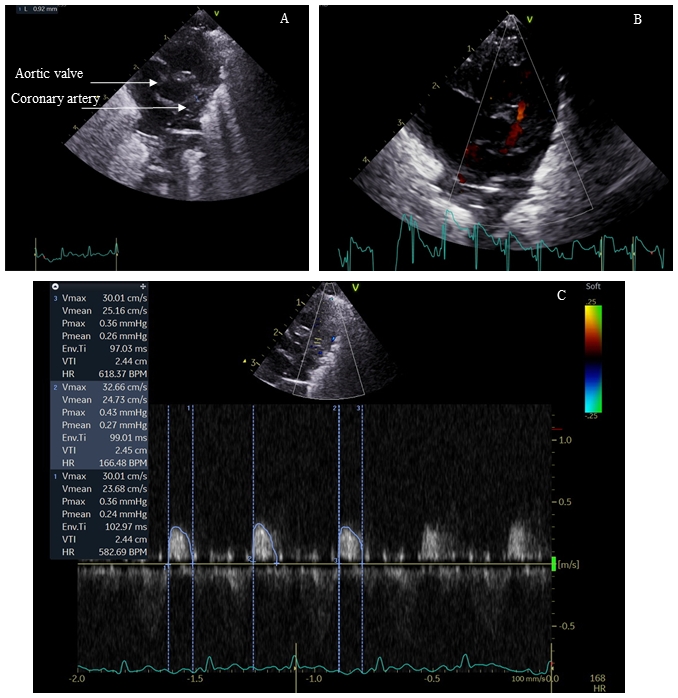
Methods: This echocardiography study compared CABF and cardiac function in preterm FGR infants, against AGA infants during the initial postnatal weeks of life. Cardiac function and CABF were measured with Vivid E95 Advantage Cardiovascular Ultrasound using a 12 Hz probe. Diastolic CABF was measured in the left anterior descending artery (main vessel supplying left ventricle [LV]), using colour Doppler flow analysis. Study was approved by the institution ethics board.
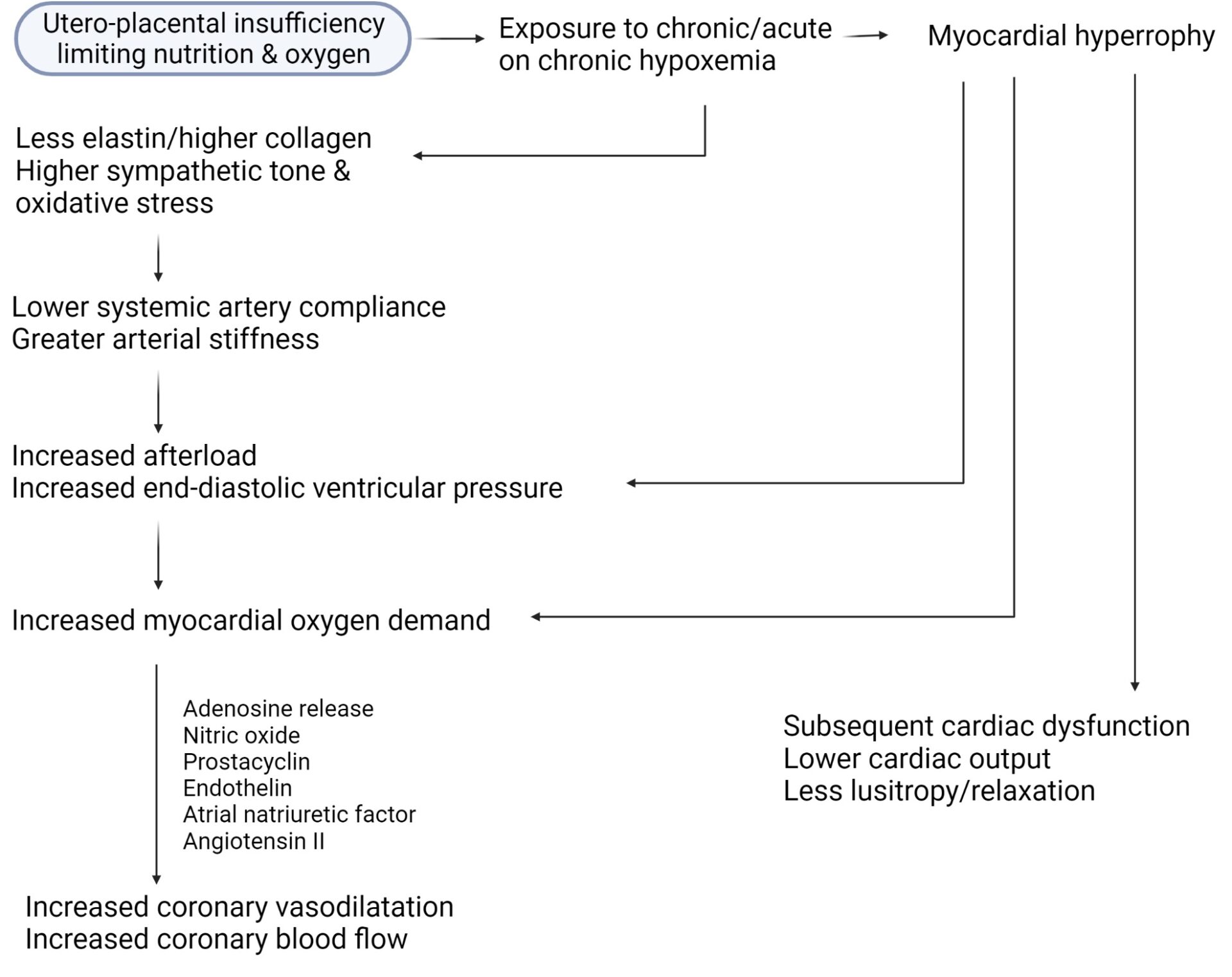
Results: Twenty eight FGR infants were compared with 26 AGA infants (gestation and birthweight, 29.7±1.3 vs 29.9±1 weeks, P=0.6 and 918±174 vs 1398±263g, P<0.001, respectively). In FGR infants, the LV was dilated and hypertrophied (↑end-diastolic diameter, posterior wall thickness and mass). The end-diastolic dimeter in FGR vs AGA infants was 14.6±9.6 vs 13.6±7.3mm, respectively, p=0.0001, and the LV mass was 6.4±0.8 vs 5.3±3g, respectively, p<0.001. Diastolic and systolic function were impaired (↑ trans-mitral E/A ratio in FGR infants; 0.84±0.05 vs 0.79±0.03, P=0.0002) and (mean velocity of circumferential fibre shortening, 1.86±0.32 vs 2.7±0.5 circ/s, P<0.001). FGR infants had higher CABF (diastolic velocity time integral, 2.4±0.9 vs 1.65±0.8cm, P=0.002). Indexing coronary flow to diastolic and systolic function noted significant differences between the groups (coronary flow: E/A [FGR vs AGA], 2.9±1.1 vs 2.1±1, P=0.01 and coronary flow: mVCFc [FGR vs AGA], 1.33±0.5 vs 0.63±0.3, P<0.001). Diastolic blood pressure was significantly higher in FGR infants (30±2 vs 25±3 mm Hg, P<0.001). Figure 1 summarizes interactions of various hemodynamic forces in the hypoxemic milieu.
Conclusions: We noted postnatal persistence of fetal circulatory adaptation even though detached from in-utero hypoxemic state. Greater CABF postnatally may reflect an altered metabolic state, fetal programming, higher prostaglandin levels in FGR, and effects of altered myocardial architecture leading to ↑ O2 consumption/requirements, possibly a combination of all the above. While coronary perfusion was higher in FGR infants (heart sparing effect), this was not accompanied by improved cardiac function, mimicking fetal age dichotomy as well the dichotomy in the cerebral circulation (increased flow [brain sparing effect] but sub-optimal neurodevelopment). Secondly, FGR cohorts might be unable to further augment CABF as per acute need (already exhausted ‘coronary flow reserve’). The failure of myocardium to relax (combined with intrinsic myocardial changes) and the coronary vascular resistance to drop in the face of increasing demand may heighten the risk of cardiovascular disease, when faced with additional workload such as strenuous exercise, obesity or hypertension. Epidemiologic studies previously demonstrated ↑ cardiovascular disease related mortality amongst adults who had low birthweight. Whether assessments of CABF in FGR-born adults provide predictive ability for CVD in middle-older ages, needs prospective study.