Ingestion of the ketone monoester (KME), thereby inducing a state of exogenous ketosis, reduces post-prandial glucose concentrations in healthy individuals1. Glucose is a principle substrate for hepatic de novo lipogenesis (DNL) with elevated availability increasing DNL2. Here we explored the hypothesis that exogenous ketosis-induced blood glucose suppression would reduce post-prandial DNL.
Ten adults free from metabolic disease (6F/4M; age: 28 ± 2 yr; BMI: 23.8 ± 1.1 kg∙m-2; mean ± SEM) underwent two 7 hr postprandial study days. After an overnight fast, participants consumed a mixed-nutrient breakfast meal that provided 2 g∙kg-1 bodyweight of carbohydrate (891.7 ± 80.9 kcal; 65.5% total kcal from carbohydrate, 10.0% protein, 24.5% fat). At 1 hr post-meal, either a KME (573 mg∙kg-1) or a taste and volume-matched placebo (PLA) drink was consumed in a blinded and randomised-counterbalanced crossover manner. For two days prior to each visit, participants undertook a eucaloric high-sugar prescribed diet (404 ± 31 g∙day-1 carbohydrate; 55.5% as sugar) to drive increased DNL. Heavy water (D2O) was consumed the evening preceding and during each study visit to achieve ~0.4% plasma enrichment. Venous blood samples were collected at fasting and for 7 hr post-prandially. Hepatic DNL was quantified as the incorporation of deuterium into newly synthesised palmitate (16:0) within very low-density lipoprotein-triglyceride (VLDL-TG), determined by gas-chromatography mass-spectrometry. Data were analysed by paired t-tests and two-way (time & condition) repeated-measures ANOVAs. All results are presented as mean ± SEM with significance at p<0.05.
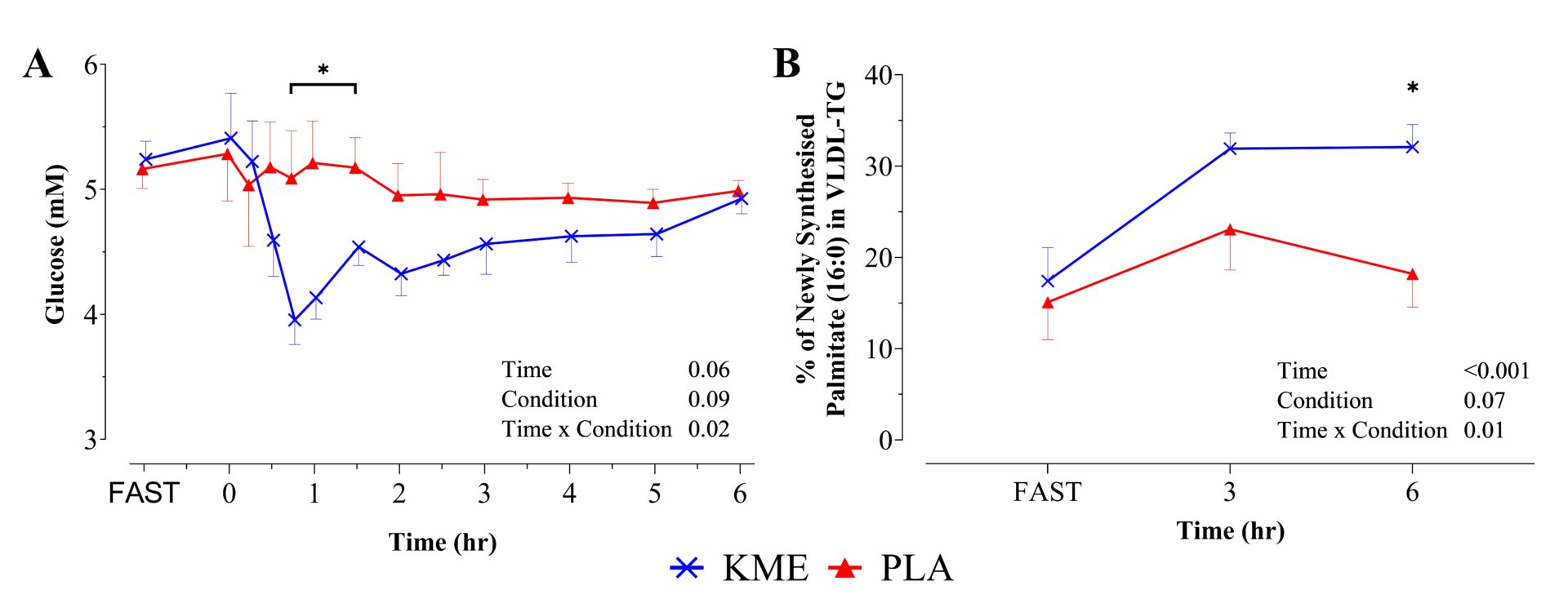
Plasma [β-hydroxybutyrate] was elevated by KME with greater concentrations observed from 15 to 300 min post-drink (p≤0.03; KME: 3.14 ± 0.42, PLA: 0.05 ± 0.01 mM). Plasma glucose concentrations were reduced after the KME drink (Fig. 1A), with area-under-the-curves 7.4% lower compared to PLA (p<0.001). Post-prandial insulinaemia was unaffected by KME. There were distinct differences in hepatic DNL, such that KME increased DNL at 360 min post-drink (absolute increase, 13.9%; p=0.01) with a tendency to also be greater at 180 min (8.8%, p=0.09; Fig. 1B). KME did not affect plasma [VLDL-TG] nor the proportion of palmitate (16:0) or other fatty acids (14:0, 16:1n-7, 18:0, 18:1n-9, 18:2n-6) within VLDL-TG (mol%). Both plasma [non-esterified fatty acid] and [glycerol] were suppressed during the latter stages of the feeding test under KME (p≤0.04). There were no differences between KME and PLA conditions for plasma [triglyceride], [cholesterol], [high-density lipoprotein], or [lactate], nor for respiratory quotient.
The current study demonstrates that post-prandial hepatic DNL is elevated under exogenous ketosis in healthy adults, despite lowered circulating glucose levels and insulin being unaffected. This might have pathological implications as Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)3 and hepatic insulin resistance4 are associated with elevated DNL. Future work investigating the influence of chronic exposure to exogenous ketosis on DNL, as well as in patient populations with MASLD and type 2 diabetes, is warranted.
Figure Caption:
FIGURE 1. A, Circulating glucose (mM). B, DNL as the proportion of newly synthesised palmitate (16:0) in VLDL-TG (%). ✱ p<0.05 between KME and PLA.