A number of sodium-dependent neutral amino acid transport systems have been functionally characterized to date, including system A, system ASC and system B0. The latter was defined from studies in the bovine renal cell line NBL-1, and unlike systems A and ASC binds aromatic amino acids such as phenylalanine, although it transports them poorly (Doyle & McGivan, 1992). An amino acid transporter cloned from the human choriocarcinoma cell line JAR was described as system B0 (ATB0, Kekuda et al. 1996) although alanine uptake into JAR cells is virtually insensitive to inhibition by phenylalanine (Torres-Zamorano et al. 1997). Here we report the cloning and characterization of a system B0 amino acid transporter from NBL-1 cells.
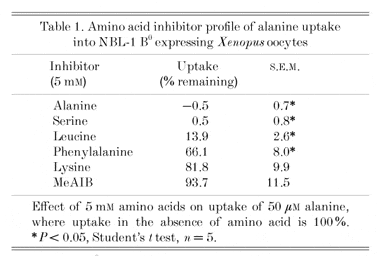
The clone isolated from NBL-1 cells (NBL-1 B0, Genbank accession AY039236) encodes a protein of 539 amino acids, which when expressed in Xenopus oocytes induces the transport of alanine (initial rate of control uptake 8.64 ± 0.48, NBL-1 cRNA injected 198 ± 16.6 pmol oocyte h-1, mean ± S.E.M., n = 5) which is sodium dependent (lithium replacement 71.9 ± 3.02). A 100-fold excess of various amino acids produces the results seen in Table 1, which are characteristic for system B0, i.e. neutral, branched and aromatic amino acids are inhibitors. The lack of effect of N-aminoisobutyric acid (MeAIB) shows that this is not system A. In NBL-1 cells, there is no inhibition of alanine uptake by glutamate or aspartate at pH 5.5, indicating that this is not system ASCT2. The inhibition of alanine uptake by phenylalanine in NBL-1 expressing oocytes was shown from a Dixon plot to be competitive (predicted Ki 1.4 mM), although phenylalanine itself is not transported. These findings are in agreement with the original characterization of system B0 in NBL-1 cells, and distinguish this transporter from the neutral amino acid transport system found in bovine renal brush-border membranes that mediates the uptake of phenylalanine from the proximal tubule (Lynch & McGivan, 1987). In addition, NBL-1 B0 activity is found on the basolateral membrane of the NBL-1 cells (Doyle & McGivan, 1992), which are more like cells from renal distal tubule.
The NBL-1 B0 clone has 82.6 % identity at the amino acid level to ASCT2 (system ASC) and 87.8 % identity to JAR B0, but there are subtle differences in the kinetics of these transporters as ASCT2 is inhibited by anionic amino acids at low pH, whereas JAR B0 is much less sensitive to phenylalanine inhibition. In conclusion, NBL-1 B0 is a new member of the superfamily of amino acid transporter genes which includes ASCT2 and ATB0, with differences in transporter phenotype brought about by intriguingly small changes in sequence. Phylogenetic comparisons of the sequences of these transporters support this view, with JAR B0 and NBL-1 B0 having more in common with each other than with ASCT2.
We thank The Wellcome Trust, MRC and Royal Society for funding these studies.