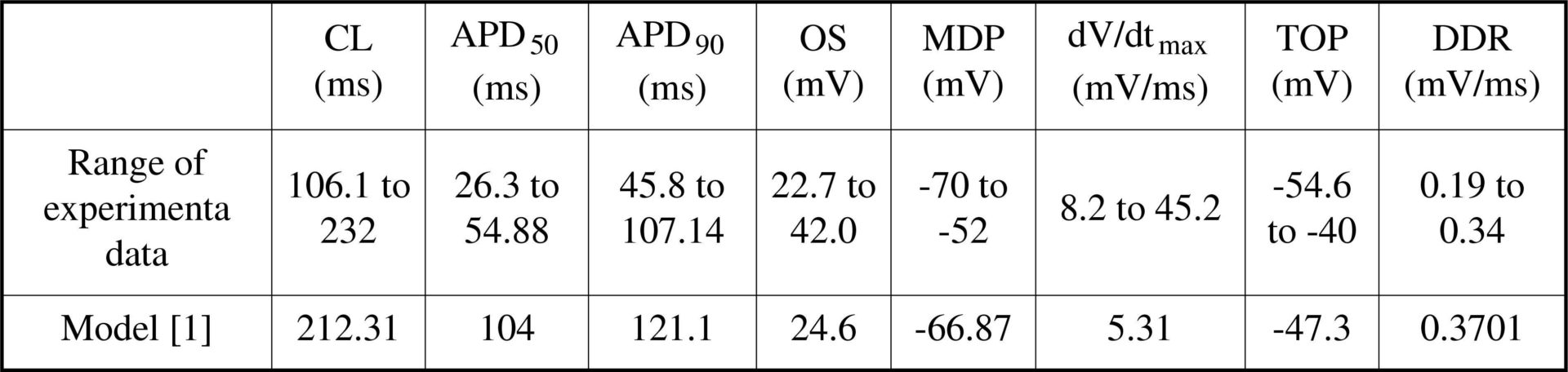
Functional roles of ion currents in the pacemaking mechanism of a mouse sino-atrial node (SAN) cell model were explored by simulating ion current blocking. In the Mangoni et al. [1] mouse SAN model, individual ionic currents were blocked to study their effects on pacemaking. Further, the constant basal [Ca2+]i was down and up regulated simulating the effects of ryadonine and caffine respectively. The effects were quantified as alterations in cell action potential (AP) characteristics, i.e., cycle length (CL), AP durations at 50% (APD50) and 90% repolarisation (APD90), overshoot potential (OS), the maximum diastolic potential (MDP), the maximal upstroke velocity (dV/dtmax), take off potential (TOP), and the diastolic depolarisation rate (DDR) [2]. Table 1 shows basal model AP characteristics and experimental data. Phase plane analysis revealed regions of the AP regulated by the respective ion currents. Diastolic depolarisation (DD) is regulated by the hyperpolarisation activated (If), sustained inward (Ist), and T-type Ca2+ (ICaT) currents acting below the basal TOP of -47 mV. AP upstroke is regulated by the fast Na+ (INa1.1 and INa1.5) and the L-type Ca2+ (ICaL1.2 and ICaL1.3) currents which act between the TOP and OS. Repolarisation of the AP is regulated by the transient outward (Ito), sustained outward (Isus), rapidly and slowly activating delayed rectifying (IKr and IKs respectively) and the inward rectifier (IK1) currents. The Na+-K+ pump (INaK) and the Na+-Ca2+ exchanger (INaCa) act as background currents in this model. Figure 1 illustrates results of the channel blocking simulation. Blocking If caused a 17.4% increase of CL due to DDR reduction. Blocking ICaT caused a 6% increase of CL. Blocking the main upstroke current, ICaL1.3, arrested pacemaking with a stable resting potential of -29.9 mV. Blocking the main repolarisation current, IKr, arrested pacemaking with a stable resting potential of -10 mV. Blocking INaCa caused an 18% reduction of CL. Reducing [Ca2+]i increased CL to 223.3 ms, a modest increase of 5.1%. A small increase of [Ca2+]i (by 20%) arrested pacemaking. The model qualitatively reproduces certain experimental observations, but Ist, INa1.1, INa1.5 are not functional. The constant intracellular ionic homeostasis rendered INaCa and INaK functionally to be background currents rather than providing coupling between intra-cellular and membrane processes, limiting their effects. The model has a limited ability to reproduce experimental results and limited predicative potential and requires further development.
University of Manchester (2010) Proc Physiol Soc 19, C99
Oral Communications: Functional Roles of Ionic Currents in A Membrane Delimited Mouse Sino-atrial Node Cell Model
S. Kharche1, M. Lei2, H. Zhang1
1. School of Physics and Astronomy, University of Manchester, Manchester, United Kingdom. 2. School of Medicine, University of Manchester, Manchester, United Kingdom.
View other abstracts by:
Figure 1. CL under ion current blocked (bl.) conditions in the SAN model [1]. Ion currents acting during DD, AP upstroke (AP-U), AP repolarisation (AP-R) and background (bac) are indicated.
Table 1. Experimental range and model AP characteristics.<#13>
Where applicable, experiments conform with Society ethical requirements.

![Figure 1. CL under ion current blocked (bl.) conditions in the SAN model [1]. Ion currents acting during DD, AP upstroke (AP-U), AP repolarisation (AP-R) and background (bac) are indicated.](https://static.physoc.org/app/uploads/2019/01/22203425/C99_A.jpg)