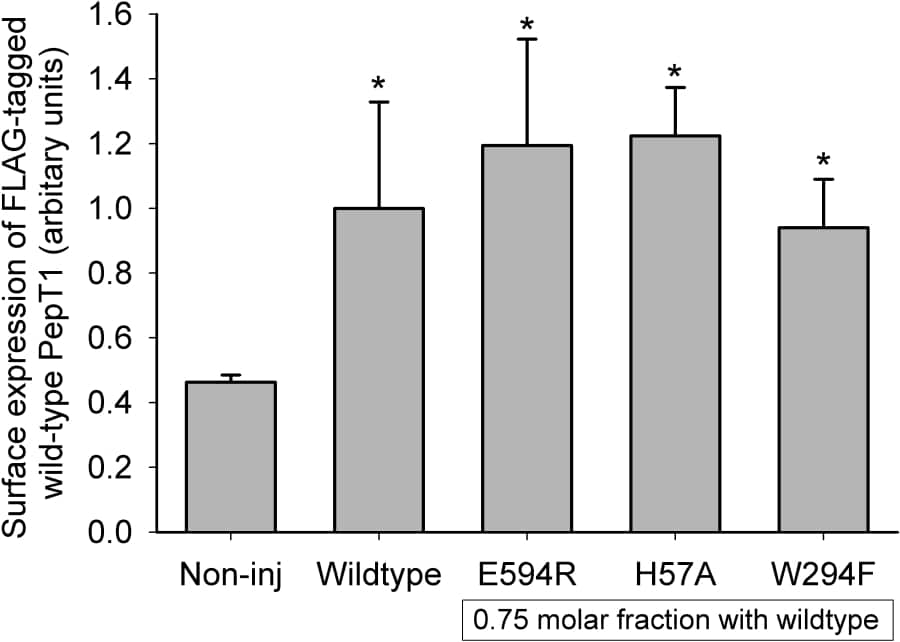
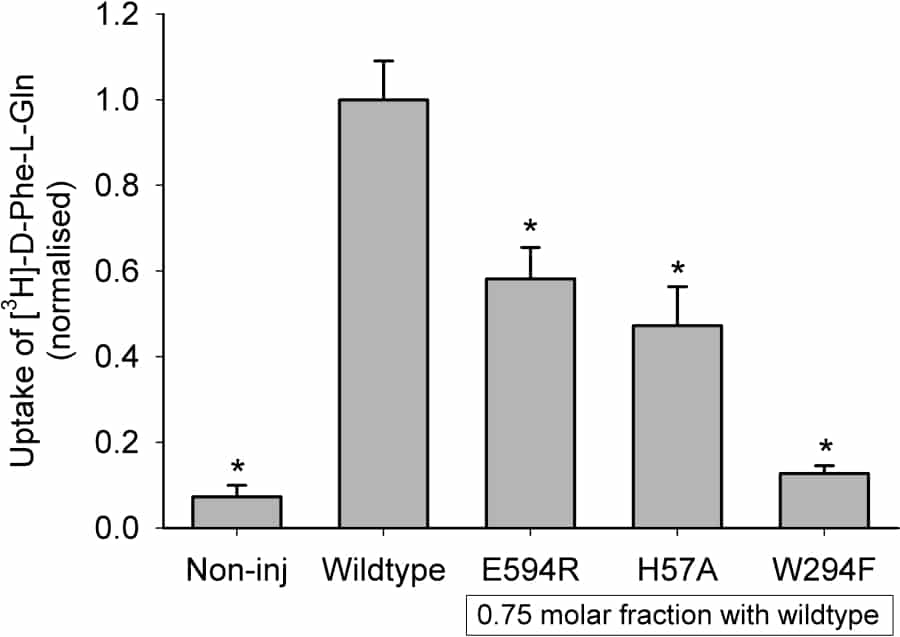
It has recently been reported that the rabbit proton-coupled peptide transporter PepT1 (SLC15a1) is a functional multimer, with a tetramer (potentially a dimer of dimers) best fitting the experimental data from co-expression of a non-functional expressed mutant W294F-PepT1 and wild-type PepT1 (Panitsas et al. 2006). The mechanism by which the mutant PepT1 inhibits the function of the wild-type PepT1 is not known. This study examines whether other non-functional expressed PepT1 mutants can also inhibit wild-type PepT1 when co-expressed in Xenopus oocytes. The mutants were in either the same region of PepT1 (E594R, transmembrane domain 10 (TMD10)) or on the opposite side (H57A, TMD2) to W294F (TMD7), based on a recent homology model (Meredith & Price 2006). The co-expression and analysis of mutant and wild-type PepT1 was as previously reported (Panitsas et al. 2006). Mutant PepT1 cRNAs were injected at a molar fraction of 0.75. The surface expression of the wild-type (FLAG-tagged) PepT1 was not affected by the co-expression of any of the non-functional mutant PepT1 proteins (Figure 1). However, the functional activity of the wild-type PepT1 was affected by the mutant PepT1 proteins (Figure 2). As had been found previously (Panitsas et al. 2006), W294F-PepT1 is very effective at inhibiting wild-type PepT1 function. The other mutants tested, E594R- and H57A-PepT1 also produced a significant reduction in the function of the wild-type PepT1, despite not affecting its expression in the oocyte plasma membrane. This study shows that the interaction of expressed non-functional PepT1 mutants is not unique to W294F-PepT1. The fact that E594R- and H57A-PepT1 were apparently equally as effective in suppressing wild-type PepT1 activity, despite their predicted positions being on opposite sides of the protein from each other (but at the same depth in the TMD) suggests that the effect on the wild-type protein might not be due to where the proteins physically interact with each other. Rather, we hypothesis that the inhibition may be due to the inability of the non-functional protein to undergo the change in shape envisaged during the translocation step in the transporter cycle, when the substrate binding site is re-orientated from facing one side of the membrane to the other.
University of Cambridge (2008) Proc Physiol Soc 11, PC168
Poster Communications: Further evidence for the interaction of rabbit PepT1 proteins when expressed in Xenopus oocytes.
S. Brown1, M. Pieri1,2, D. Meredith2
1. Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom. 2. School of Life Sciences, Oxford Brookes University, Oxford, United Kingdom.
View other abstracts by:
Figure 1: Luminometry results for FLAG-tagged wild-type PepT1 expression in oocytes in the absence and presence of mutant PepT1. Data are mean ± SEM for ≥2 oocyte preparations with ≥20 oocytes per condition in each. *p<0.05 Student’s t-test versus non-injected control oocytes.
Figure 2: Uptake results for FLAG-tagged wild-type PepT1 expressed in oocytes in the absence and presence of mutant PepT1. Data are mean ± SEM for ≥2 oocyte preparations with 10 oocytes per condition in each. *p<0.05 Student’s t-test versus FLAG-tagged wild-type PepT1.
Where applicable, experiments conform with Society ethical requirements.