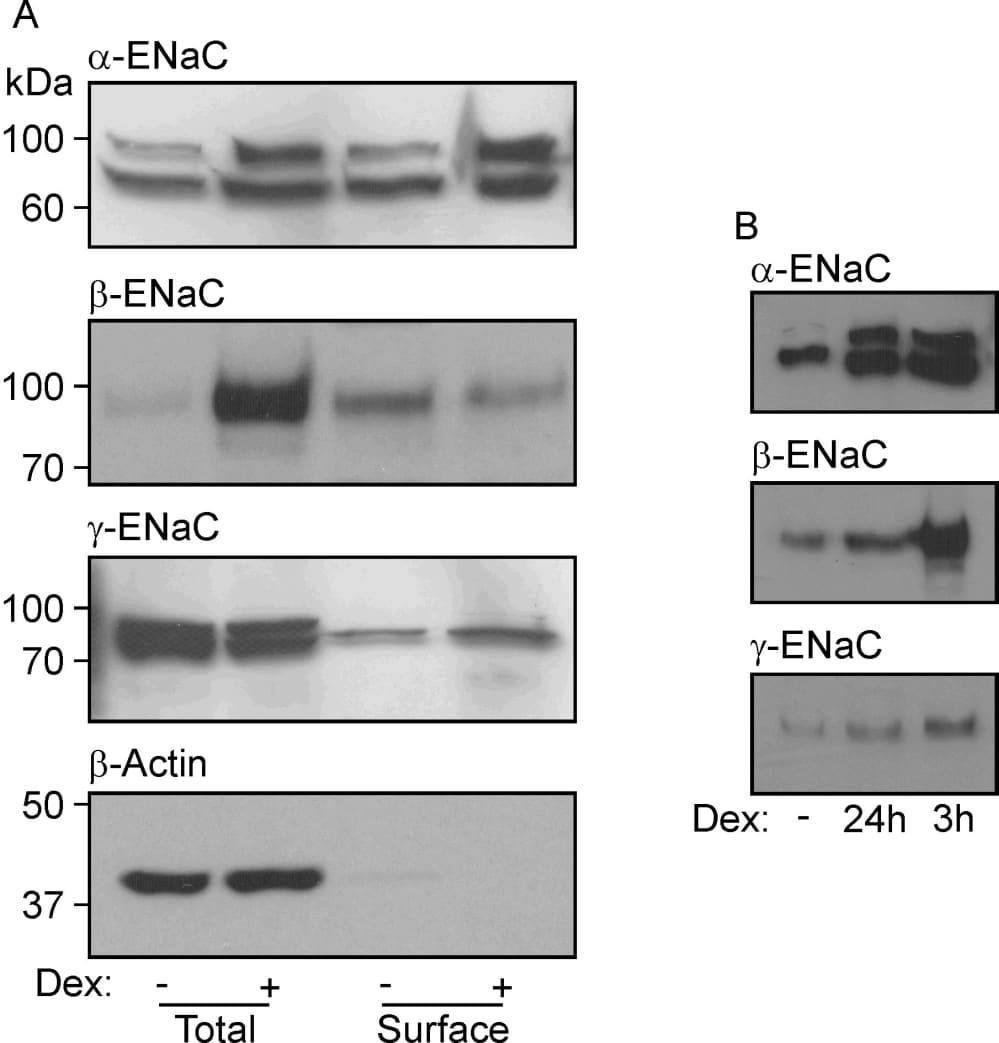
Glucocorticoids control epithelial Na+ conductance via a mechanism dependent upon serum and glucocorticoid-inducible kinse 1 (SGK1) a kinase thought to control the surface abundance of α-,β- and γ-ENaC. However, whilst dexamethasone activates SGK1 in H441 cells, this response is rapid (1 – 3 h) and transient whilst the induction of Na+ current is relatively slow (> 3 h) and sustained (Watt et al. 2010). We have therefore explored the effects of dexamethasone upon α-,β- and γ-ENaC abundance in the total and in surface protein pools; surface proteins were isolated by biotinylation (10mM sulfosuccinimidyl-2-(biotinamido)-ethyl-1,3’dithioproprionate, 1 h 4°C) / streptavidin binding. Two α-ENaC isoforms (~100 kDa and ~77 kDa) were detected in total and surface pools and dexamethasone (0.2 µM, 24 h) consistently increased the abundance of each isoform in both protein pools (Fig. 1A). Beta-ENaC was present as a single band (~100 kDa) and, whilst dexamethasone clearly increased the overall expression of this subunit, it had no effect upon surface abundance. Although two γ-ENaC isoforms (96 kDa, 76 kDa) were detected in total protein, only one band (~80 kDa) was present at the cell surface and dexamethasone had no effect upon the abundance of this surface-exposed protein (Fig. 1A). Each ENaC subunits is therefore present at the surface of glucocorticoid-deprived cells, and the fact that such cells do not display Na+ currents (Watt et al. 2010) cannot, therefore, be attributed to the absence of α-,β- and γ-ENaC from the membrane. Moreover, the induction of ENaC activity by prolonged (~24 h) dexamethasone stimulation is not associated with any change to the surface expression of β- and γ-ENaC whilst the effect on α-ENaC can be explained by an increase in the overall expression level (Fig1 A). Since glucocorticoid-induced SGK1 activity peaks after ~3 h (Watt et al. 2010), subsequent experiments compared the abundance of α-,β- and γ-ENaC at the surface of hormone-deprived cells, and cells exposed to 0.2 µM dexamethasone for 24 h or 3h. As anticipated (see above) 24 h stimulation increased the surface expression of α-ENaC with no effect upon β- or γ-ENaC (Fig. 1B). Increases in the surface abundance of α-,β- and γ-ENaC were, however, evident in cells exposed to dexamethasone for only 3 h (Fig. 1B), despite the fact that such cells do not express discernible Na+ currents (Watt et al. 2010). Whilst periods of SGK1 activation are associated with the recruitment of α-,β- and γ-ENaC to the cell surface, such co-ordinated increases in the surface expression of these channel subunits cannot explain the SGK1-dependent activation of ENaC in these cells (Watt et al. 2010).
Durham University (2010) Proc Physiol Soc 21, C18 and PC18
Oral Communications: Glucocorticoids cause only a transient increase in the surface abundance of the epithelial Na+ channel (ENaC) subunits (╬▒-,╬▓- and ╬│-ENaC) in H441 human airway epithelial cells
N. Ismail1, S. C. Land1, S. M. Wilson1
1. Centre of Cardiovascular and Lung Biology, University of Dundee, Dundee, United Kingdom.
View other abstracts by:
Figure 1. (A) Western blots showing the abundances of α-, β- and γ-ENaC and β-actin in total and surface-exposed proteins extracted from cells that had maintained (24 h) in hormone-free medium or in medium containing 0.2 µM dexamethasone. The lack of β–actin from the surface pool confirms that this protein preparation is essentially devoid of intracellular proteins. Essentially identical results were obtained in 5 experiments. (B) Abundances of α-, β- and γ-ENaC in surface exposed proteins isolated from glucocorticoid-deprived cells and from cells at identical passage that had been exposed to 0.2 µM dexamethasone for 3 h or 24 h (n = 3).
Where applicable, experiments conform with Society ethical requirements.