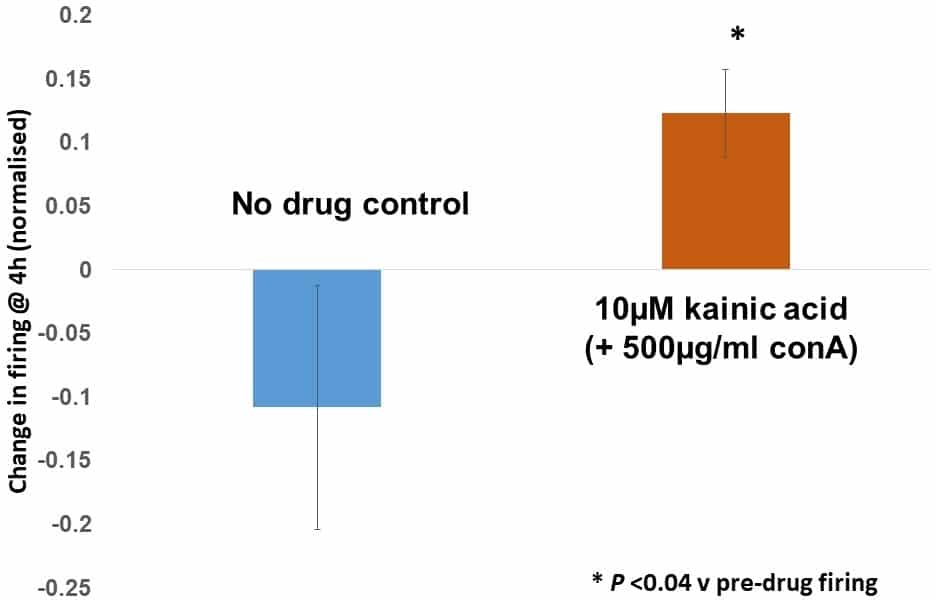
We are targeting baroreceptors as a promising new drug target for treating high blood pressure (hypertension). Hypertension is the world’s most common killer disease (WHO), due to its contribution to cardiovascular disease, stroke and kidney failure, which account for >7m deaths p.a.. Current treatments are inadequate: most patients need multiple drugs either to treat the initial condition or the resulting side-effects. Even then >10% of patients are unresponsive and there have been no better drugs against existing targets for over 20 years. Thus, new drugs and therapeutic targets are needed. Baroreceptors are such a novel and highly effective target. Electrical stimulation of the aortic depressor nerve (ADN) in drug-resistant patients reduces blood pressure by >20 mmHg, and can even replace medication. However, the surgery is invasive, expensive, challenging and only 50% of patients are anatomically suitable. We propose a more inclusive pharmaceutical approach, by targeting a system we discovered that modulates mechanosensory terminal sensitivity via an atypical glutamate receptor linked to PLD activation1,2, which we recently reported as GluK23,4. We are pharmacologically characterising the efficacy of targeting this receptor in isolated aorta-ADN preparations as a translational step towards clinical application. ADN-aorta preparations from humanely killed (ASPA, 1986) adult Sprague-Dawley rats (200-400 g) were maintained in a bath perfused with oxygenated artificial CSF. The aorta was inflated to just below firing threshold then pulsed above it at 10 min intervals or continuously at ~1 Hz. The effects of glutamate receptor and candidate transducer channel ligands on firing responses were analysed automatically using custom written Matlab codes. Data are presented as normalised mean ± SEM, with significance threshold for differences being P = 0.05, using a paired t-test. Kainic acid (10 µM) robustly increased firing in the presence of concanavalin A (500 µg/ml) to block glutamate receptor desensitisation. The enhancement has slow onset (initiates 90 min after addition) and persists >1 h after wash-out. Normalised to the pre-drug control level, kainic acid increased firing +14.5 ± 3.5% (P <0.04, n=4) while controls showed a non-significant decrease (-12.1 ± 9.8%, P >0.31, n=6), a difference of >25%. The TRP channel blocker 2-APB strongly inhibited pressure-evoked firing at 100 µM (-76.9 ± 10.7% of control, P <0.02, n=3) while 10 µM had no effect. Similarly, the DEG/ENaC channel blocker amiloride (1 mM) markedly decreased firing (-84.1% ± 17.5% of control at 3 h, P <0.05, n=3). These data show baroreceptor firing can be pharmacologically controlled and the GluR-mediated enhancement offers a novel approach to raising baroreceptor firing, and hence lowering blood pressure, which may have important clinical implications. The effects are slow onset (~90 min) but long lasting. Further experiments are planned to fully characterise this response.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, PC210
Poster Communications: Glutamate receptor and transducer channel modulation of baroreceptor firing in a rat isolated aorta/aortic depressor nerve preparation.
C. Giuraniuc1, R. W. Banks2, J. F. Paton3, G. S. Bewick1
1. Institute of Medical Sciences, University of Aberdeen, Aberdeen, Scotland, United Kingdom. 2. Department of Biosciences, University of Durham, Durham, United Kingdom. 3. Department of Physiology, University of Auckland, Auckland, New Zealand.
View other abstracts by:
10 μM kainate (with concanavalin A to block receptor desensitisation) significantly increased baroreceptor firing relative to pre-drug levels (P <0.04, n=4), while no drug control preparations underwent an overall, but non-significant, decrease (P=0.31, n=6).
Where applicable, experiments conform with Society ethical requirements.