Introduction: Non-alcoholic fatty liver disease (NAFLD) is an independent risk factor for heart disease (1). Hepatic HIF2α deletion, is protective against NAFLD in some rodent models (2), but the effects on basal or stimulated cardiac function are unknown.
Objective: To determine cardiac function ex vivo in Western diet-induced NAFLD and the impact of hepatocyte-specific HIF2α deletion in mice.
Methods: HIF2α was deleted in hepatocytes using Cre-recombinase under control of the albumin promoter in male HIF2αfl/fl mice. These mice (hHIF2α-/-), and related male, Cre-negative (WT) mice were fed a high fat, fructose and cholesterol Western diet (WD, Research Diets D09100310), or chow for 28 weeks (n = 4-5 per group). Cardiac basal systolic and diastolic function, and responsiveness to the acetylcholine receptor agonist carbachol (range: 10-8-10-6 M) and the β-adrenergic receptor agonist isoprenaline (range: 10-9-10-7 M) were assessed in isolated, Langendorff-perfused hearts. Sympathetic dominance was calculated as the ratio of left ventricular (LV) developed pressure (LVDP) responses to maximum doses of isoprenaline and carbachol. Data were analysed by two-way ANOVA with Tukey’s post hoc test for significant genotype:diet interactions, and presented as mean ± standard deviation.
Animal procedures were approved by the University of Cambridge Animal Welfare and Ethical Research Board and conducted according to UK Home Office regulations under the Animals in Scientific Procedures Act (1986).
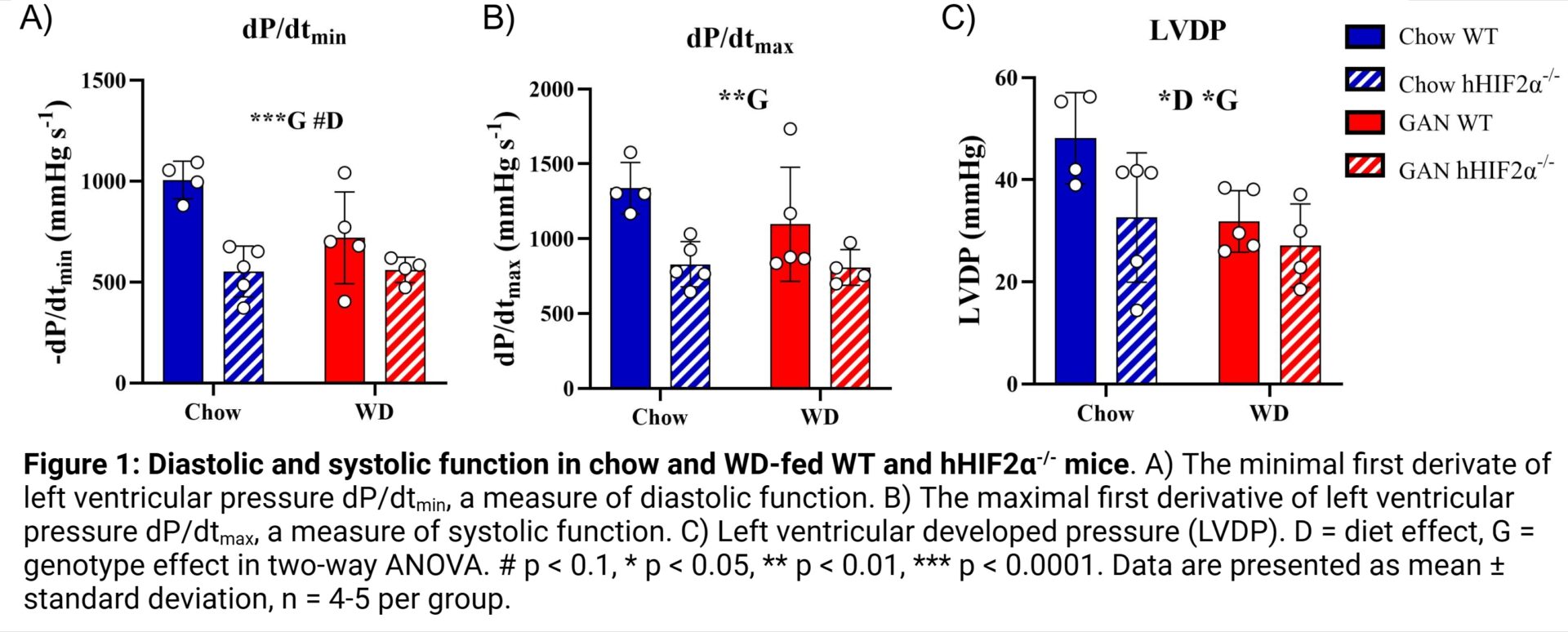
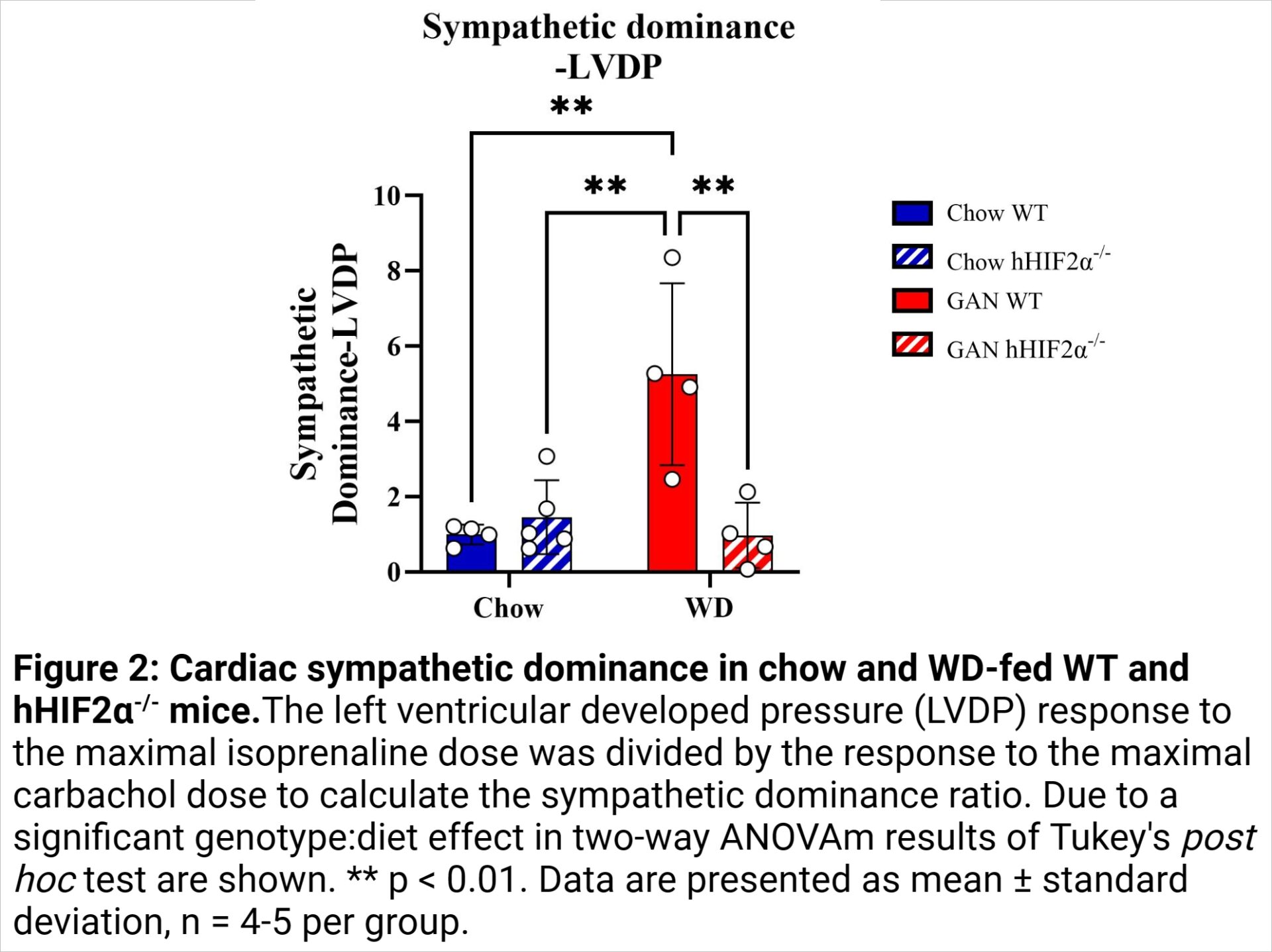
Results: Three indices of basal cardiac dysfunction occurred in hHIF2α-/- mice relative to WT mice. The minimum first derivative of LV pressure (dP/dtmin), a measure of diastolic function, was 34% lower in hHIF2α-/- mice than WT mice (p = 0.0007; 1006 ± 93 mmHg.s-1 in chow-fed WT mice, 720 ± 228 mmHg.s-1 in WD-fed WT mice, 552 ± 126 mmHg.s-1 in chow-fed hHIF2α-/- mice, 561 ± 63 mmHg.s-1 in WD-fed hHIF2α-/- mice) (Fig. 1A). Similarly, the maximum first derivative of LV pressure (dP/dtmax), a measure of systolic function, was 32% lower in hHIF2α-/- mice (p = 0.0035; 1338 ± 172 mmHg.s-1 in chow-fed WT mice, 1097 ± 381 mmHg.s-1 in WD-fed WT mice, 830 ± 151 mmHg.s-1 in chow-fed hHIF2α-/- mice, 808 ± 119 mmHg.s-1 in WD-fed hHIF2α-/- mice) (Fig. 1B). LVDP, another measure of systolic function, was 25% lower in WD-fed mice (p = 0.0278) and 24% lower in hHIF2α-/- mice (p = 0.0384; 48 ± 9 mmHg in chow-fed WT mice, 32 ± 8 mmHg in WD-fed WT mice, 33 ± 13 in chow-fed hHIF2α-/- mice, 27 ± 8 mmHg in WD-fed hHIF2α-/- mice) (Fig. 1C). In contrast, relative to chow-fed WT mice (ratio = 1.0 ± 0.3), the sympathetic dominance ratio, a measure of cardiac stimulated function, was 5.3-fold higher (5.3 ± 2.4) in WD-fed WT mice but normalised in WD-fed hHIF2α-/- mice (Fig. 2).
Conclusion: The data provide novel insight into liver-heart crosstalk. Hepatocyte-specific deletion of HIF2α was associated with basal cardiac dysfunction, but protection against cardiac sympathetic dominance in the mouse heart. Since cardiac sympathetic dominance has been linked to cardiac arrhythmia and heart failure (3), further investigation of hepatic HIF2α deletion may suggest strategies for cardiac therapy in NAFLD.