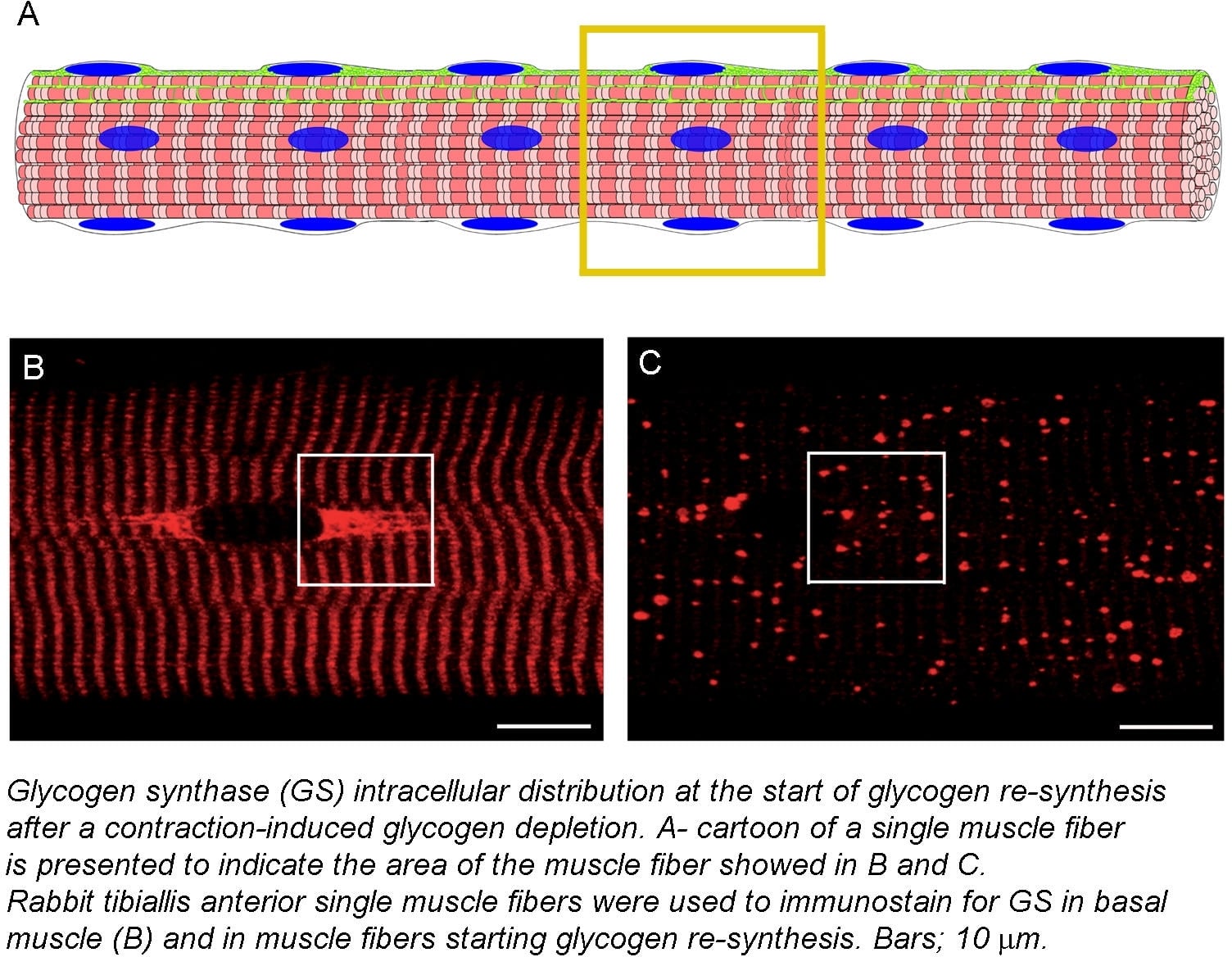
Skeletal muscle is not only essential to maintain body posture and perform physical activity, skeletal muscle is also indispensable to maintain metabolic body homeostasis, also referred to as metabolic health (for review see (1). Initially, skeletal muscle metabolism was mainly interesting for exercise physiologists. During the last decades, however, research resources invested into understanding skeletal muscle metabolism have drastically grown due to its involvement in the development of insulin resistance and type 2 diabetes mellitus (T2DM). In spite of this, very basic metabolic pathways, such as glycogen and lipid metabolism, and their regulation by exercise and insulin, remain elusive. I believe one of the reasons is that most of our knowledge comes from studies performing in vitro measurements of muscle homogenates. Skeletal muscle is a heterogeneous tissue, where apart from muscle fibers, that have distinctive functional and metabolic properties (for review see (2)), other cell types are also present (satellite cells, adipocytes, macrophages and endothelial cells, among others). In consequence, biochemical measurements performed on muscle homogenates are informative, but sometimes misleading. With the development of new tools and methods to study skeletal muscle morphology, it has become obvious that metabolic processes and signaling pathways are highly compartmentalized events. During the last years, our work has identified some of the mechanisms that regulate skeletal muscle glycogen and lipid metabolism by redistribution of enzymes between intracellular compartments (3;4;5), and it has also shown that transverse tubules play a key role in insulin signaling transduction (6;7). Furthermore, the use of a new method to metabolically characterize skeletal muscle fibers has revealed the existence of two clearly distinctive pools of type I muscle fibers. In order to understand cellular processes, we need to investigate them in situ and in selected cell types. We need to be aware of the heterogeneity and organization of the studied tissue, and acknowledge the importance of intracellular compartmentalization as a regulator of cellular processes.
University of Manchester (2010) Proc Physiol Soc 19, SA64
Research Symposium: Heterogeneity and intracellular compartmentalization of skeletal muscle metabolism
C. Prats1
1. Biomedical Sciences, Copenhagen University, Copenhagen, Denmark.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.