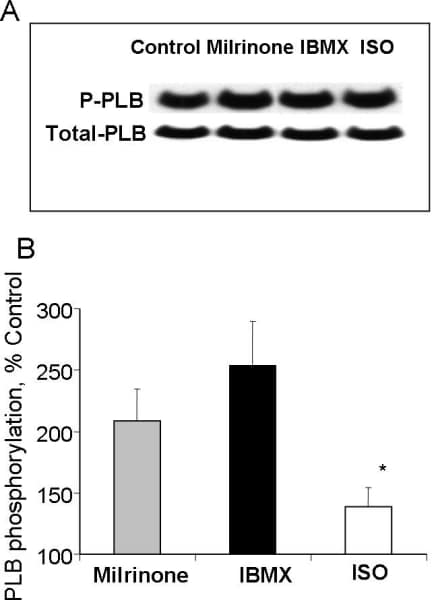
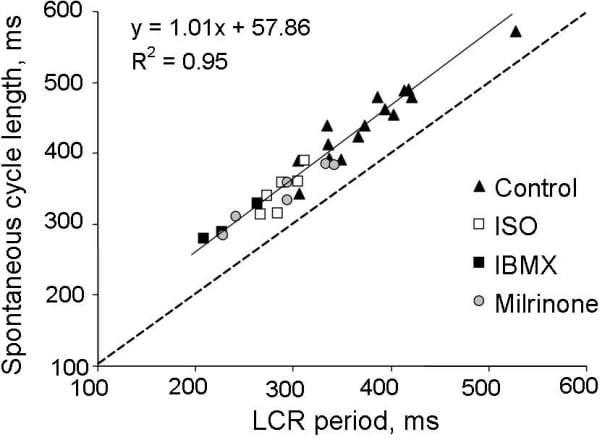
Rabbit sinoatrial node cells (SANC) possess localized, submembrane Ca2+ releases (LCR) via ryanodine receptors (RyR) which occur during a late part of the diastolic depolarization (DD)(1). Regardless of size, SANC exhibit intense RyR, Na+-Ca2+ exchanger (NXC) and SERCA2 labeling and submembrane NCX/RyR colocalization (2). Spontaneous, roughly periodic LCR activate inward NCX current, accelerate the nonlinear DD rate and thus control the SANC spontaneous beating rate (1,3). The basal cAMP content of isolated SANC homogenates is markedly increased compared to that in ventricular myocytes, and LCR periodicity in SANC is precisely controlled by cAMP PKA-dependent phosphorylation of Ca2+ cycling proteins (4). This high basal level of cAMP is required for spontaneous beating and is due to constitutively activated adenylate cyclases (ACs) in SANC, since specific AC inhibitor, MDL-12,330A (400 μmol/L) markedly reduces cAMP content and the same concentration of MDL stops spontaneous beating (4). We hypothesized that a high level of cAMP in SANC might be a result of low cAMP degradation by phosphodiesterases (PDEs). Spontaneously beating SANC were isolated from adult rabbit hearts, according to the NIH ethical requirements. To study Ca2+ cycling in SANC we employed confocal microscopy with Fluo-3 as a Ca2+ indicator. We simultaneously recorded spontaneous SANC action potentials (perforated patch-clamp) and line-scan images beneath sarcolemma. The cAMP content of isolated SANC homogenates was assessed by radioisotope assay. Phospholamban (PLB) phosphorylation was measured by Western blotting of SANC homogenates with antibodies to total PLB and phosphorylated PLB at Serine 16. To test our hypothesis we employed a broad-spectrum PDE inhibitor, IBMX. Contrary to our expectations, IBMX (100 μmol/L), induced 9-fold increase in the cAMP level and ~55% increase in the SANC firing rate (from 145 ± 8 to 221 ± 6 beat/min, n=10) demonstrating a high basal activity of PDEs in SANC. The PDE superfamily consists of 11 families and PDEs 1- 5 are present in the heart (5). Using specific inhibitors against different PDE subtypes we determined which PDE subtype relate to an increase in the spontaneous SANC beating rate. A suppression of PDE3 by milrinone (50 μmol/L) produced 47% increase in the beating rate (from 145 ± 13 to 207 ± 10 beat/min, n=9), i.e., similar to the effect of IBMX, and exceeding effects of a saturating concentration of β-AR agonist isoproterenol (33%, n=9). The effects of other PDE inhibitors on the spontaneous beating rate were relatively small. Suppression of either total PDE activity by IBMX or PDE3 activity by milrinone markedly increased cAMP PKA-dependent PLB phosphorylation (Fig. 1), and this was reversed by a specific PKA inhibitor peptide, PKI. Additionally, milrinone produced ~46% increase in the amplitude of L-type Ca2+ current from 13.3 ± 1.6 in control, to 19.0 ± 2.2 pA/pF with milrinone (n=8, P<0.05). The positive chronotropic effect of PDE inhibition is critically dependent upon subsarcolemmal RyR Ca2+ release during the late DD. Specifically, either IBMX or milrinone decreased the LCR period (the interval from a prior AP induced SR Ca2+ transient to subsequent LCR occurrence), increased the LCR amplitude (P<0.01) and size (P<0.01) leading to earlier and augmented LCR Ca2+ release. This PDE inhibition-induced reduction in the LCR period was greater than that produced by isoproterenol. The LCR period following IBMX, milrinone or ISO was highly correlated with the concomitant decrease in the SANC spontaneous cycle length (Fig 2). When RyRs were disabled by ryanodine, both IBMX and milrinone failed to amplify LCRs, to accelerate the DD rate and to increase the SANC firing rate, despite preserved PDE inhibition induced augmentation of L-type Ca2+ current. Thus, high basal constitutive PDE activity in SANC coexists with constitutively active AC, providing a negative feedback on the latter to confine cAMP level. Constitutively active PDEs, via reduction in cAMP-mediated, PKA-dependent protein phosphorylation prolong the local RyR Ca2+ release period to control the basal spontaneous SANC beating rate.
University of Manchester (2007) Proc Physiol Soc 8, SA16
Research Symposium: High constitutive activities of both adenylyl cyclases and phosphodiesterases regulate local subsarcolemmal RyR Ca2+ release to control normal sinoatrial node cell automaticity
T. M. Vinogradova1, A. E. Lyashkov1, Y. Li1, S. Sirenko1, A. Younes1, E. G. Lakatta1
1. NIA, NIH, Baltimore, MD, USA.
View other abstracts by:
Figure 1. A, representative Western blot of the basal level of PLB phosphorylation at serine16, and total PLB in SANC prior to and following milrinone (50 μmol/L), IBMX (100 μmol/L) or β-AR stimulation (1 μmol/L, ISO). B, relative values of phosphorylated PLB normalized to total PLB (n=8).
Figure 2. The effects of PDE inhibition (IBMX, 100 μmol/L or milrinone, 50 μmol/L) or β-AR stimulation (isoproterenol, 0.1 μmol/L) to alter the spontaneous cycle length are linked to their ability to control the LCR period.
Where applicable, experiments conform with Society ethical requirements.