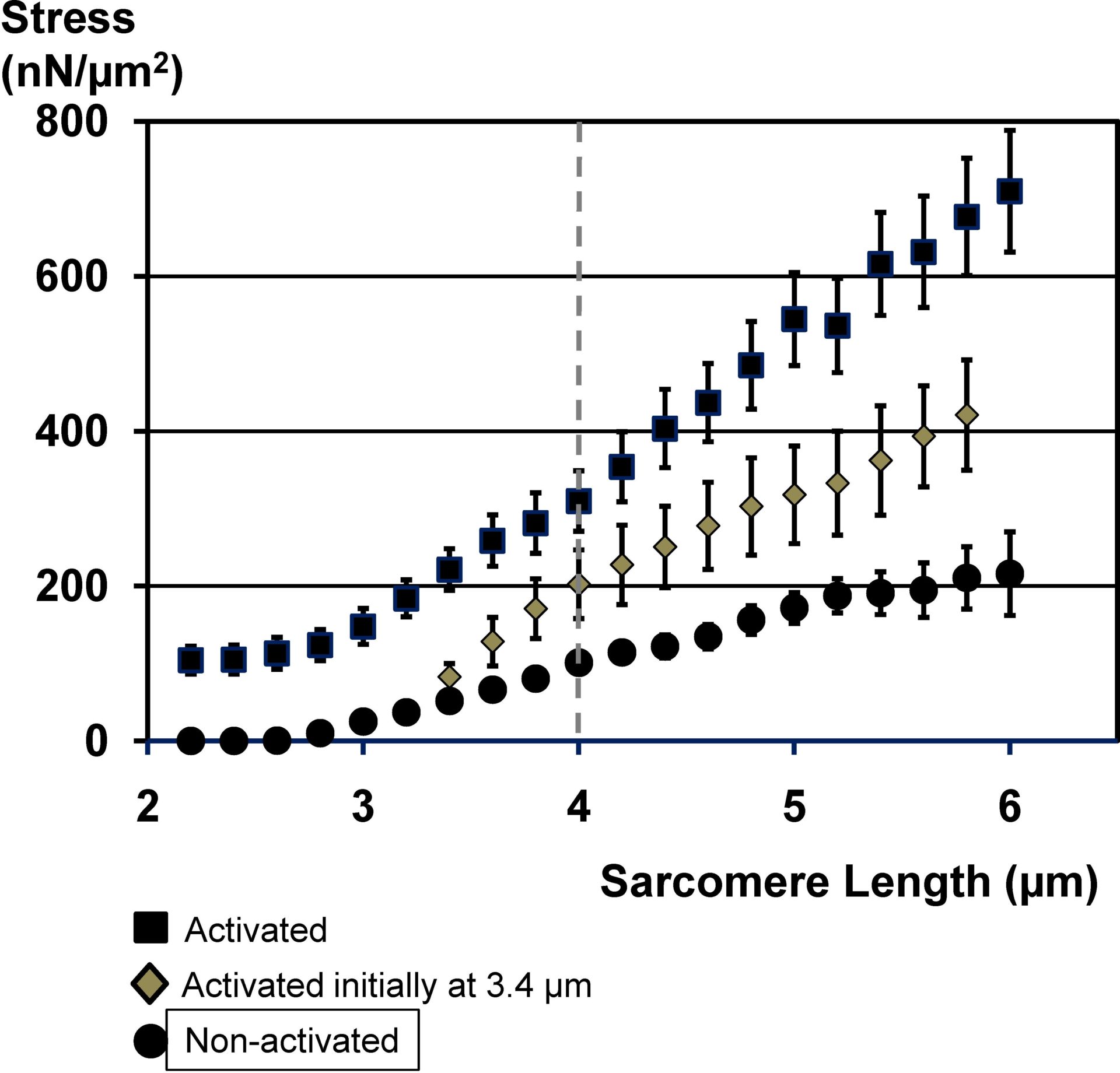
When a muscle is actively stretched (shortened) its steady-state force following the stretch (shortening) is increased (decreased) relative to the purely isometric force at the same length. This history dependence of muscle force production was first described systematically more than half a century ago (1), but cannot be explained by the reigning paradigm of muscle contraction: the cross-bridge theory (2, 3). For the past three decades, history dependence had been explained with structural non-uniformities; specifically the development of sarcomere length non-uniformities when muscles were stretched (shortened) actively on the “unstable” descending limb of the force-length relationship (4, 5). The sarcomere length non-uniformity theory allowed for precise predictions, including that force enhancement following an active stretch cannot occur on the ascending part of the force-length relationship and that the enhanced forces can never exceed the maximal isometric forces at obtained on the plateau of the force-length relationship. However, these two basic predictions were shown to be not satisfied in a series of experiments from different laboratories (e.g. 1, 6). Recently, we discovered that passive forces following an active stretch of muscles, fibres and myofibrils were increased (7). When eliminating titin from isolated myofibril preparations, this passive force enhancement was abolished indicating that titin might play a force regulatory role. Stretching troponin C depleted myofibrils (to inhibit cross-bridge connections between the contractile proteins actin and myosin) in solutions of increasing calcium concentration resulted in an increase in passive forces, suggesting that titin is a molecular spring whose stiffness can be modulated by calcium (e.g., 7). Unfortunately, the increase in force associated with titin’s calcium sensitivity only accounted for a few percent of the observed increases in passive force with active muscle stretching. When stretching single myofibrils passively (low calcium concentration) and actively (high calcium concentration) beyond actin-myosin filament overlap, forces in the actively stretched condition were 3-4 times greater at lengths where cross-bridge forces were absent, and these differences reached values approximately 2-3 times the maximum active isometric force at the plateau of the force-length relationship. How can such high forces be explained in the absence of actin-myosin based cross-bridges forces? When eliminating titin, these force difference are abolished. Calcium activation alone (when cross-bridge attachments are inhibited) merely accounts for a tiny amount of the observed force increases. However, when myofibrils are actively stretched from different parts of the descending limb of the force-length relationship, and thus from different force levels, the increase in force beyond actin-myosin filament overlap is proportional to that force (Figure 1). From these results we conclude that titin is a strong regulator of force in skeletal muscle and becomes particularly important at long sarcomere lengths. Titin’s force regulation depends on the amount of active force, but is essentially independent of calcium concentration. We tentatively suggest that titin’s force regulation is caused by a force-dependent interaction of titin with actin which causes the free spring length of titin to become smaller thereby increasing its stiffness, and thus force upon stretching.
University of Manchester (2010) Proc Physiol Soc 19, SA12
Research Symposium: History dependence of muscle force production: structural non-uniformities, cross-bridge action or something altogether different?
W. Herzog1
1. Kinesiology, University of Calgary, Calgary, Alberta, Canada.
View other abstracts by:
Figure 1: Mean (±1SE) stress (force/area) of single myofibrils as a function of average sarcomere lengths for myofibrils stretched in an activating solution from optimal length (high calcium concentration - activated square symbols), or stretched actively from an average sarcomere length of 3.4µm (high calcium concentration - diamonds), and stretched in a non-activated state (low calcium concentration - circles). Note that the forces beyond actin-myosin filament overlap (beyond about 4.0µm (vertical line) are dramatically greater for the actively compared to the passively stretched myofibrils, even though actin-myosin based cross-bridge forces cannot account for these differences at these sarcomere lengths.
Where applicable, experiments conform with Society ethical requirements.