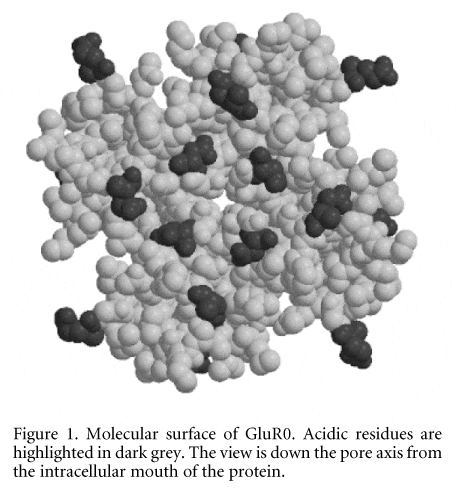
Ionotropic glutamate receptors (iGluRs) mediate excitatory synaptic events in the central nervous system of vertebrates. iGluRs and K+ channels are thought to share a common transmembrane (TM) topology in their core pore-forming domains (Wood et al. 1995). A prokaryotic K+-selective glutamate receptor (GluR0) has significant homology with the bacterial K+ channel KcsA (Chen et al. 1999). Therefore, the structure of the pore-forming domain of KcsA may be used as a template for homology modelling the equivalent domain in GluR0. A homology model of the transmembrane region of GluR0 was generated using Modeller6 based on the X-ray structure (2.0 ü) of KcsA (Zhou et al. 2001). Two initial models were generated, based on slightly different (in the loop/P-helix region) sequence alignments. On the basis of initial molecular dynamics (MD) simulations, the model that gave the lowest structural drift was selected for further analysis. This model was used as the starting point for further analysis and for MD simulations. The distribution of charged residues in GluR0 (see Fig. 1) compared with that in KcsA indicates a similarity between the models.
The GluR0 model was inserted into a pre-equilibrated octane slab (a mimic of a lipid bilayer) and the system was solvated and counter-ions added to ensure overall electroneutrality. The positioning of K+ in the crystal structure of KcsA was replicated in our model. The protein and ions were then subjected to a protein-restrained MD run of 400 ps after which an unrestrained production simulation was run for 6 ns. Structural drift of the GluR0 model from its initial conformation and structural fluctuations assessed over the course of the simulation revealed no significant fluctuations in the filter and the TM helices, in particular the M2 and P helix. This is consistent with hydrogen-bond analysis, which revealed long-lived interactions involving residues in close-proximity to the filter region and the existence of four inter-subunit salt bridges (Asp213-Arg217) also near the filter region. Similar interactions are observed in KcsA (Asp80-Arg89) and are thought to maintain the relative rigidity of the filter, which is an essential component for K+ selectivity. The concerted single-file motion of K+ within the filter of GluR0 was similar to that observed in KcsA simulations (Sansom et al. 2000). Analysis of the filter region revealed minor structural fluctuations during the concerted motion of ions, which also correlates well with the behaviour of the filter in KcsA. This homology modelling and simulation study of GluR0 provides an insight into the dynamic behaviour of K+-selective GluR0. A number of similarities between KcsA and GluR0 were revealed. In particular, this study has indicated that the M1, P and M2 pore-forming regions in GluR0 and KcsA have similar architectures and possibly similar functional roles.
This work was supported by the MRC, Wellcome Trust and OSC.