Collagen is the most abundant protein in the human body, fulfilling a wide range of mechanical and structural roles. Despite our considerable knowledge of its biochemistry and cell biology, very little is known about the physiology of collagen. Using a novel method to directly measure collagen synthesis using stable isotope tracers (Babraj et al. 2002), we have previously demonstrated that collagen in human bone, tendon, ligament and muscle are synthesized at rates which are much faster than hitherto expected (Babraj et al. 2003) and that muscle collagen (mostly endomysium) shows remarkable acute anabolic responses to strenuous exercise with a pattern and extent similar to that of human myofibrillar protein. We therefore hypothesized that as myofibrillar protein is also acutely stimulated by feeding protein or amino acids, both muscle and bone collagen synthesis might also be.
We studied four groups each of 4 healthy young men, (all 29 ± 3.5 y, BMI 24.3 ± 2.1, kg/m2 mean ± S.E.M., with no significant differences between the groups). The studies had the approval of the Tayside Ethics Committee. In two groups of subjects we measured muscle myofibrillar protein synthesis and collagen synthesis and in two groups we measured bone collagen synthesis in the post absorptive and fed states. Subjects in the muscle group were given an infusion of octreotide and insulin throughout to maintain insulin (~10 mIU/l) and glucose (~4-5 mM) at the post-absorptive values and either ingested 20 g of essential amino acids or remained post-absorptive. Subjects in the bone group were either given a venous infusion of glucose, lipid and amino acids (equivalent to 1 X basal metabolic rate, calculated using Harris-Benedict equation) or remained post-absorptive. Quadriceps muscle and iliac crest were biopsied using the conchotome and bone needle techniques applied under local anaesthesia (1 % lignocaine). Bone collagen synthesis was measured, using established methods (Babraj et al. 2002) as the incorporation over 2 h of [1-13C]proline applied as a flooding dose. Muscle collagen synthesis was measured as the incorporation over 3 h of [1-13C]keto-isocaproic acid applied as a primed constant infusion.
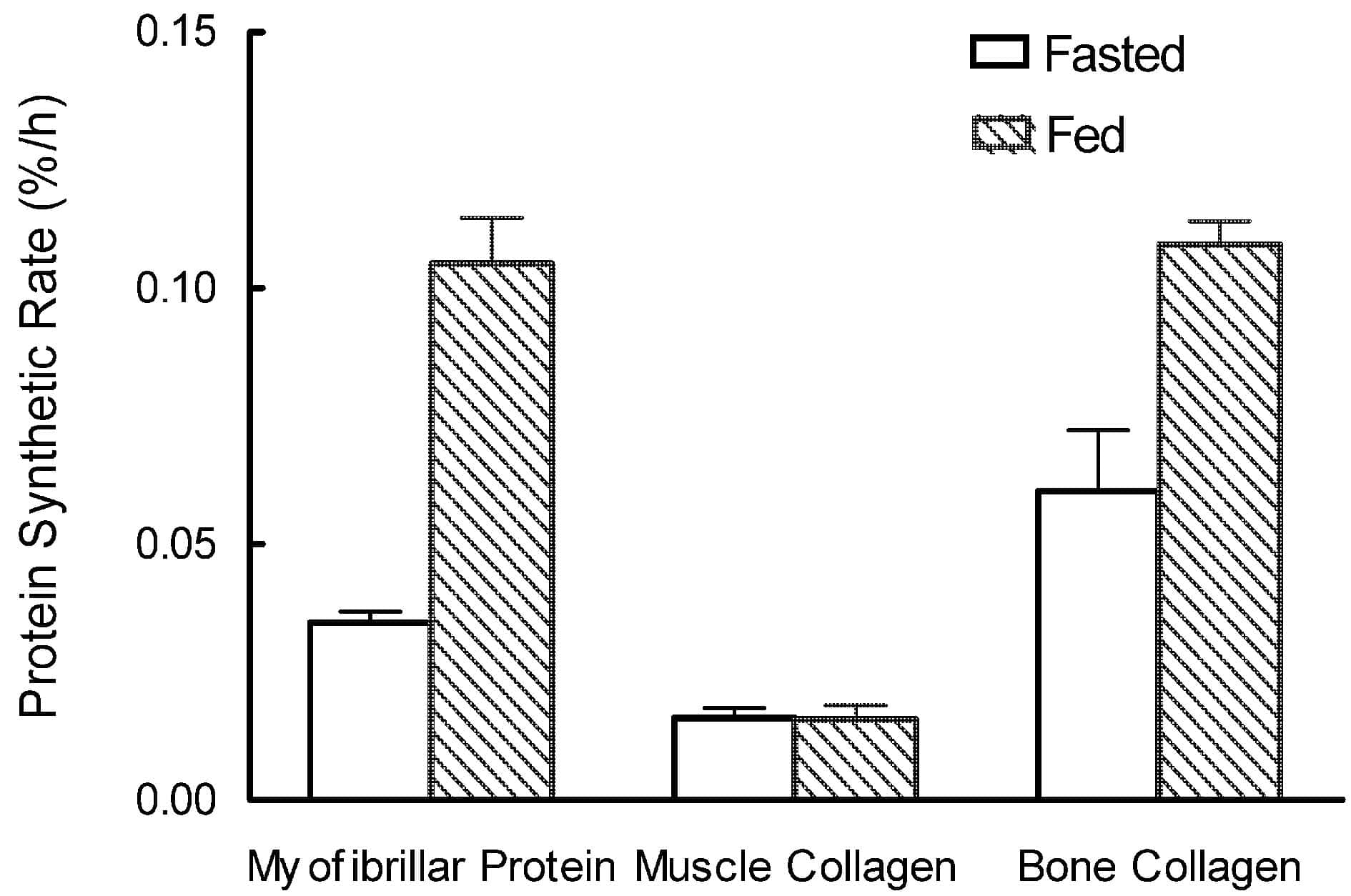
Although myofibrillar protein responded as expected, rising from 0.035 ± 0.002 to 0.105 ± 0.009 %/h within 3 h of feeding, muscle collagen synthesis was unchanged at 0.016 ± 0.002 %/h. In bone, however, the synthetic rate of the hot water extractable fraction (which contains 90 % of bone collagen) rose from 0.060 ± 0.012 to 0.108 ± 0.004 %/h on feeding.
The results suggest that nutritional modulation of bone collagen synthesis is rapid and marked, features which are likely to be of importance in the growth and maintenance of bone mass and bone quality.
This work was supported by The Wellcome Trust and UK Medical Research Council