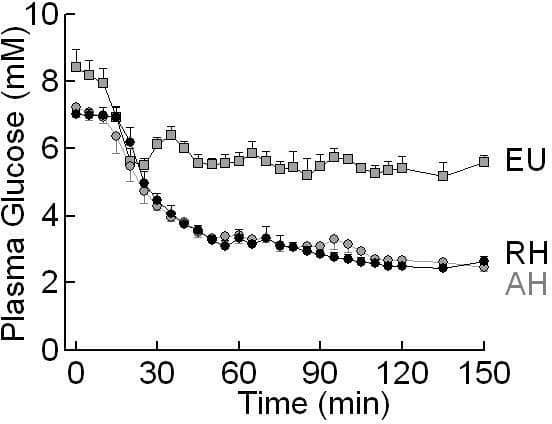
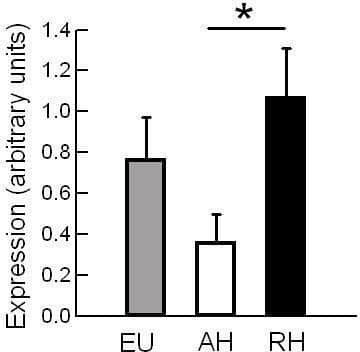
*Joint 1st author Counterregulatory responses (CRR) to acute hypoglycaemia may become impaired following exposure to recurrent hypoglycaemia. Current evidence suggests that the predominant sites for hypoglycaemia sensing and/or triggering CRR are in the hypothalamus. We hypothesised that impaired CRR develops through coordinated changes in gene expression in hypothalamic pathways. We created a rodent model of impaired CRR in catheterised (inserted surgically under isoflurane anaesthesia 1 week prior to studying) Sprague Dawley rats. One group (recurrent hypo [RH], n = 4) underwent 3 days of sc regular insulin injections (10, 8 and 6 units/kg on days 1 to 3) followed by a day 4 hyperinsulinaemic (20 mU/kg/min) hypoglycaemic clamp. A second group (acute hypo [AH], n = 4) had 3 days of sc saline followed by a day 4 hypoglycaemic clamp, while a third control group also had 3 days sc saline followed by clamped euglycaemia ([EU], n = 4). At 150 min, whole hypothalami were removed, frozen for later RNA extraction etc and expression analysis using Affymetrix rat 230 genome 2.0 chip. Genes showing significant change (using Genespring GX- Agilent) were subjected to pathway analysis (Ingenuity Systems). We identified 116 and 103 genes which significantly increased or decreased expression respectively in AH compared with EU, a surprisingly high number considering that these groups were treated identically except for 120 min of exposure to different plasma glucose values on day 4 (fig below). Pathway analysis suggested significant decreases (p<0.05) in AH in glucocorticoid (GC), interleukin (IL) 6 and acute phase response signalling. Looking then at the comparison between acute and recurrent hypoglycaemia (AH vs RH), 143 genes increased and 158 decreased. As a striking comparison when compared with AH, pathways with significant increases in RH (p<0.05) included GC, IL-6, IL-10, IL-2 and acute phase response pathways. Finally, given this analysis suggested coordinated changes in hypothalamic cytokine/ inflammatory signalling and previous data suggesting a role in the hypothalamus for the key inflammatory cytokine IL 1-beta in controlling peripheral metabolism, we quantified IL-1B expression with RT-PCR. In keeping with the broad patterns seen in microarray data, hypothalamic IL1B expression was significantly higher in RH than AH (figure below). In summary, our data show that (1) even 120 min of hypoglycaemia results in robust coordinated changes in hypothalamic gene expression and (2) that marked differences exist in gene expression between acute and recurrent hypoglycaemia in cytokine/ inflammatory pathways. We speculate that that non- neuronal cells- astrocytes and/or microglia- may contribute to modulating neuronal glucose-sensing in the hypothalamus by locally modulating cytokine/ inflammatory signalling.
University of Oxford (2008) Proc Physiol Soc 12, PC7
Poster Communications: Hypothalamic cytokine signalling pathways increase following recurrent hypoglycaemia
S. Moore*1, M. A. Osundiji*1, T. Adeloye1, D. Lam2, J. Shaw1, C. Riches1, P. Markkula1, L. K. Heisler2, G. Yeo1, M. Evans1
1. Institute of Metabolic Science, University of Cambridge, Cambridge, United Kingdom. 2. Dept of Pharmacology, University of Cambridge, Cambridge, United Kingdom.
View other abstracts by:
Plasma Glucose during Day 4 Clamp Studies
Hypothalamic IL-1B Expression
Where applicable, experiments conform with Society ethical requirements.