Introduction: Vascular cognitive impairment (VCI) is the second most prevalent for of dementia and is associated with reduced cerebral blood flow. We know this causes hypoxia inducible factor (HIF) expression and harnessing this could represent a new therapeutic target for VCI. Prolyl-hydroxylase domains (PHD) increase HIF expression; DMOG and FG4592 are PHD inhibitors. We aim to characterise additional mechanisms of action.
Methods: Neuronal cell-line rat pheochromocytoma (PC12 cells) were cultured with D5H5B media at 37Ëš C with 21% O2 and 5% CO2. DMOG, FG4592 (100uM) or 1% DMSO (vehicle) was added across four oxygenation conditions: 21% O2, 21% O2 and glucose deprivation (GD), 1% O2, and 1% O2 glucose deprivation (OGD) for a duration of 6 or 24-hours. Each experiment consisted of three biological and three technical replicates. 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromine (MTT) and lactate-dehydrogenase (LDH) assays were carried out to determine energy production and cytotoxicity. HIF-1α and HIF-2α protein levels were examined by Western immunoblotting while HIF-1α and PHD2 RNA expression was also analysed via q-PCR.
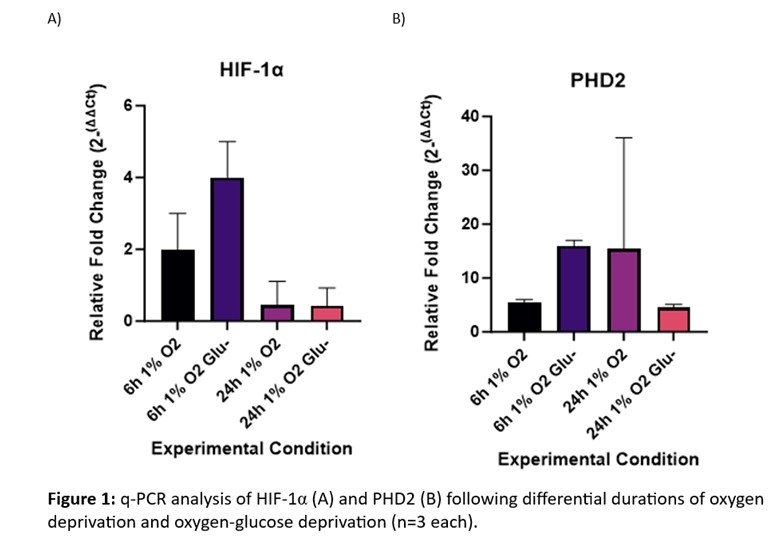
Results: Relative HIF1α RNA expression was increased following oxygen deprivation, and this was exacerbated with OGD at 6-hours but was not observed at 24-hours (Figure 1A). Interestingly, PHD2 RNA expression was only increased with OGD exposure at 6-hours, but the inverse was observed at 24-hours exposure (Figure 1B), though high variability was observed with the OGD group.
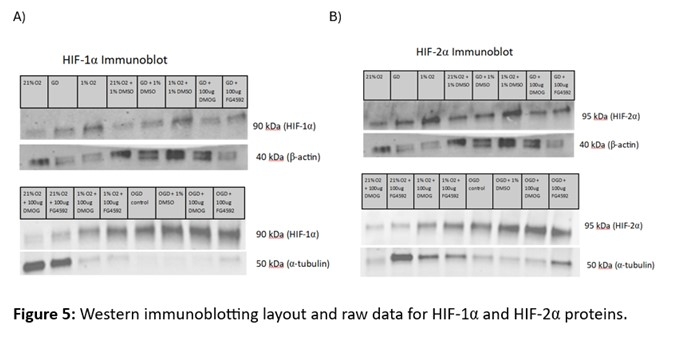
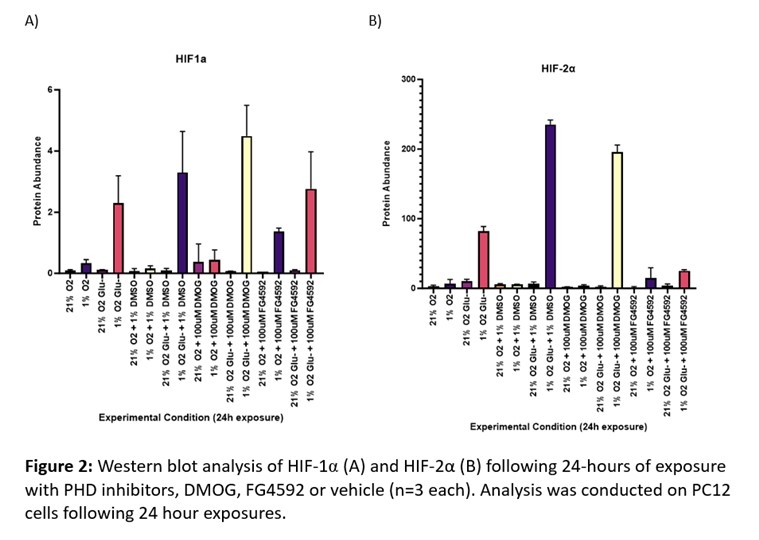
As expected, under typical oxygenation conditions at 24-hours HIF-1α protein expression was low, but it only slightly increased when oxygen was withdrawn. Expression levels increased dramatically during OGD and the same was true under vehicle conditions and with DMOG. However, there was an increase in expression when oxygen was withdrawn and FG4992 was provided (Figure 2A).
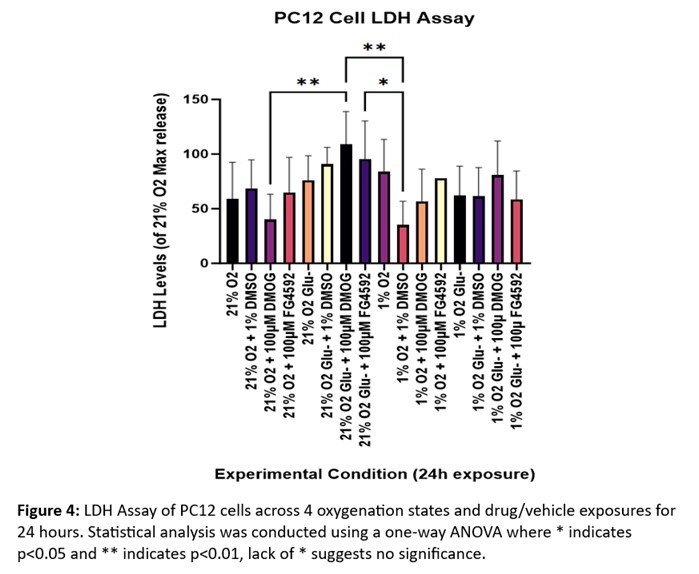
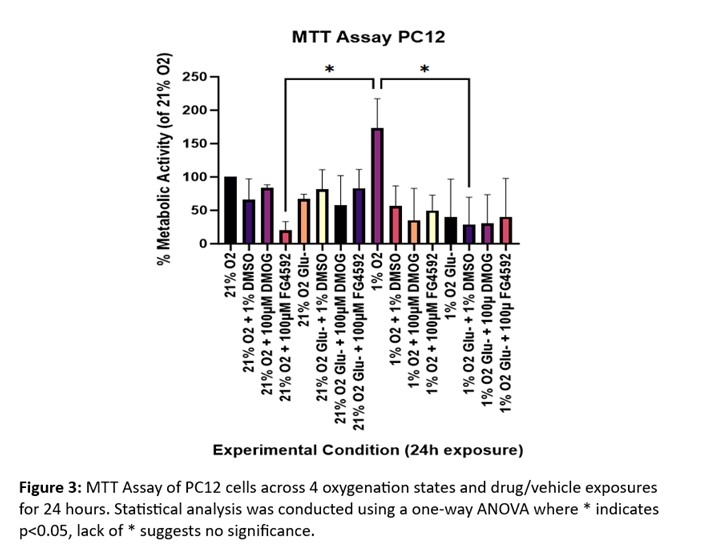
Decreased metabolic activity is presented across all OGD exposures when compared to 1% O2, thus highlighting the role of glucose in cellular metabolism (Figure 3). Paired with LDH release to assess cytotoxicity, an increase in cytotoxicity is observed in GD exposed cells with FG4592 administration (Figure 4).
Conclusions: HIF signalling increases following OGD exposure due to PHD inactivation, thus limiting HIF degradation and increasing its abundance. With the inhibition of PHD by DMOG administration, we see a further increase in OGD HIF abundance, thus supporting the role of DMOG in HIF therapeutics. Regarding PHD2 expression at an RNA level, it is possible that a negative feedback-loop is in play by which increased HIF-1α expression causes a further increase in PHD2 expression to counteract this signalling alteration. The pathway by which DMSO causes increased HIF signalling remains unclear but poses useful insights for future research.
Deoxygenation causes PHD expression downregulation to therefore allow the activation of HIF, which aligns with these results. When paired with FG4592, we see further increase in HIF signalling as this increases the deregulatory effects of PHD inactivation to form a summative HIF abundance response. Further research is required to understand the precise mechanisms at play for the signalling pathways of DMOG and FG4592. Furthermore, western immunoblotting for HIF 6-hour exposures and PHD2 will be carried out and paired with q-PCR data for HIF-2α.