Introduction: Overweight and obesity are characterised by accumulation of excess adiposity and a subsequent systemic, chronic, low-grade inflammation, which is associated with a range of metabolic disorders including dyslipidaemia and hyperglycaemia (Calder et al., 2011). Our recent meta-analysis suggested that supplementation with carnosine, or its rate-limiting precursor β-alanine, reduces fasting glucose and HbA1c in humans and rodents with overweight, obesity, or diabetes (Matthews et al., 2021). The primary aim of the study was to assess the feasibility and acceptability of chronic β-alanine supplementation in adults with overweight or obesity; with a secondary aim to explore the effect of supplementation on markers of glycaemic control.
Methods: Thirty participants with overweight or obesity (BMI: ≥25 to <40 kg/m2; n=9 with prediabetes: HbA1c 42-47 mmol/mol) were randomised (stratified for age, sex, BMI, and HbA1c) to receive 4.8 g/day of sustained-release β-alanine (n=14, age 57 ± 11y, BMI 30.6 ± 2.9 kg/m2, HbA1c 39.5 ± 4.0 mmol/mol) or a matched-placebo (n=13, age 59 ± 10y, BMI 31.6 ± 3.0 kg/m2, HbA1c 40.2 ± 4.6 mmol/mol) for 3-months. Twenty-seven participants completed the trial. Adherence (1-month, 2-months, follow-up) and side effects (baseline, acute response to supplementation, 1-month, 2-months, and follow-up) were recorded. Fasting blood samples were taken using venepuncture from the ante-cubital fossa at baseline and follow-up, and biomarkers of glycaemic control were analysed: HbA1c, glucose, insulin, C-peptide, homeostatic model assessment (HOMA) of β-cell function (HOMA2-%B) and insulin resistance (HOMA-IR). Ethical approval was granted by Nottingham Trent University and NHS Health Research Authority (REC reference: 21/NW/0280) research ethics committees, in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Data were analysed using a Bayesian approach involving informative and non-informative prior distributions, and estimation of posterior probabilities. Descriptive statistics are presented as mean ± 1SD; inferential data presented as median [95% credible intervals (CrI)]. Trial preregistration: NCT05329610.
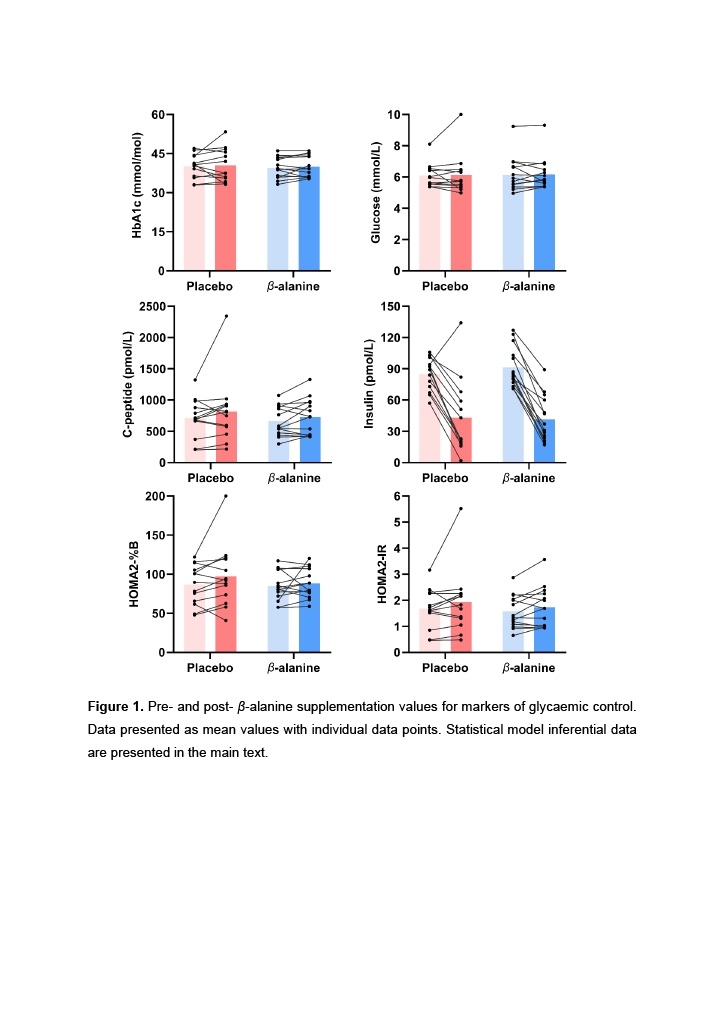
Results: Feasibility outcomes: high-dose β-alanine was well-tolerated over 3-months; evidenced by high adherence rates (β-alanine: 0.92 [0.85 to 0.95], placebo: 0.91 [0.84 to 0.95]), low attrition (6.7% and 13.3%), and participant-reported side effects which remained below baseline values and comparable across groups. Exploratory outcomes: results did not show clear evidence in favour of or against supplementation for glycaemic control outcomes: HbA1c (-0.06 [-0.30 to 0.15 mmol/mol]), fasting glucose (-0.02 [-0.50 to 0.45 mmol/L]), C-peptide (-30.1 [-211.7 to 151.3 pmol/L]), insulin (-7.4 [-15.3 to 0.32 pmol/L]), HOMA2-%B (-5.5 [-14.3 to 3.5]), or HOMA-IR (-0.18 [-0.48 to 0.11]) (Figure 1).
Conclusion: The present study shows that 3-months of β-alanine supplementation is well-tolerated and does not cause adverse events in adults with overweight and obesity. We did not find clear evidence that supplementation affected markers of glycaemic control; however, our feasibility estimates suggest that a fully powered study to detect meaningful effects would need to be large. Researchers should consider alternative approaches or more advanced conditions, such as prediabetes or type-2 diabetes.