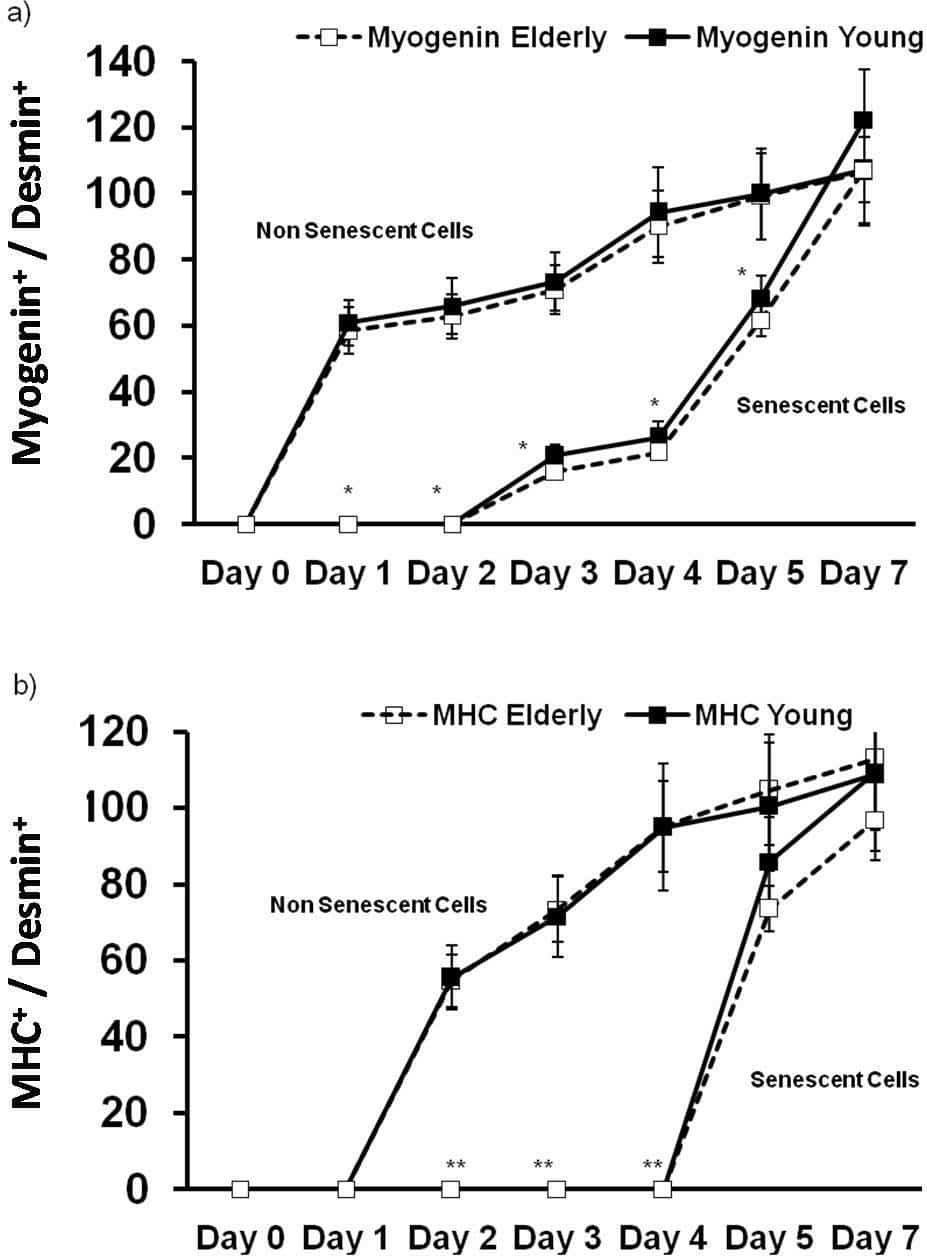
Replicative senescence of cells in culture has been used as a model to study the ageing process in human muscle (e.g. Bigot et al., 2008). However, the relevance of this approach to in vivo ageing remains unclear. The aim of this study was to investigate if in vivo muscle ageing differs from in vitro muscle ageing by studying the progression of cell differentiation in primary human muscle cells obtained from young and elderly people. Muscle biopsy samples were taken under local anaesthesia (2% lignocaine) from the vastus lateralis muscles of 4 young (3 male and 1 female aged, 23-25) and 4 elderly (1 male and 3 female aged, 67-82) subjects. Myoblasts (satellite cells) were isolated, expanded in culture (3-4 passages) and stored in liquid N2. Following thawing cells were cultured in a skeletal muscle cell growth medium (GM) to 50% confluence and plated in 96 well dishes at a density of 7000 cells/well. For the in vivo ageing study the GM was replaced after 24 hours with a differentiation medium (DM) and cultured for a further 7 days in DM, with aliquots of cells being fixed every 24 hours in 4% paraformaldehyde + 0.2% triton. For the in vitro ageing study the cells were continually passaged in GM until they reached replicative senescence. Senescence was defined as failure to divide for three consecutive weeks. The senesced cells were then similarly cultured in DM for 7 days. In both experiments, cells expressing markers of differentiation (myogenin and myosin heavy chain) were identified using immunocytochemistry, counted and expressed relative to the total cell content. The proportion of desmin positive cells (myoblasts) prior to differentiation was also determined. The data were analysed using a three way mixed factorial ANOVA or a one way ANOVA where appropriate and P <0.05 was accepted as the level of significance. A lower proportion of desmin positive cells was observed in the senesced compared to the non-senesced cells (82+8% v 32+3%, n=8, p<0.01). When normalised to the initial desmin content, no difference was observed in the proportion of cells obtained from the young and elderly subjects that expressed the two markers of differentiation at any time point. This was the case in both the senesced and non-senesced cells (Figure 1a,b). However, the senesced cells showed a slower time course of differentiation when compared to the non-senesced cells as evidenced by a similar proportion of differentiated cells at day 7, but significantly lower proportions of myogenin positive (days 1-5) and MHC positive (days 2-4) cells at the earlier time points. The data suggest that replicative senescence, but not in vivo ageing results in a delay in myoblast differentiation. However, it remains to be determined the extent to which this effect may have been influenced by a lower starting desmin content in the senesced cell cultures.
University of Manchester (2010) Proc Physiol Soc 19, PC161
Poster Communications: In vitro not in vivo ageing affects the differentiation of human myoblasts
M. Alsharidah1, V. Cristiana1, T. George1, N. R. Lazarus1, S. D. Harridge1
1. Division of Applied Biomedical Research, King's College London, London, United Kingdom.
View other abstracts by:
Figure1. Time course of (a) myogenin and (b) myosin heavy chain (MHC) expression, in human myoblasts obtained from young (n=4) and elderly (n=4) subjects. Cells were either differentiated shortly after culture (in vivo ageing model) or following passaging to replicative senesce (in vitro ageing). Significantly different when compared to non senescent cells (*p<0.05, **p<0.01). Data are mean value ± SEM.
Where applicable, experiments conform with Society ethical requirements.