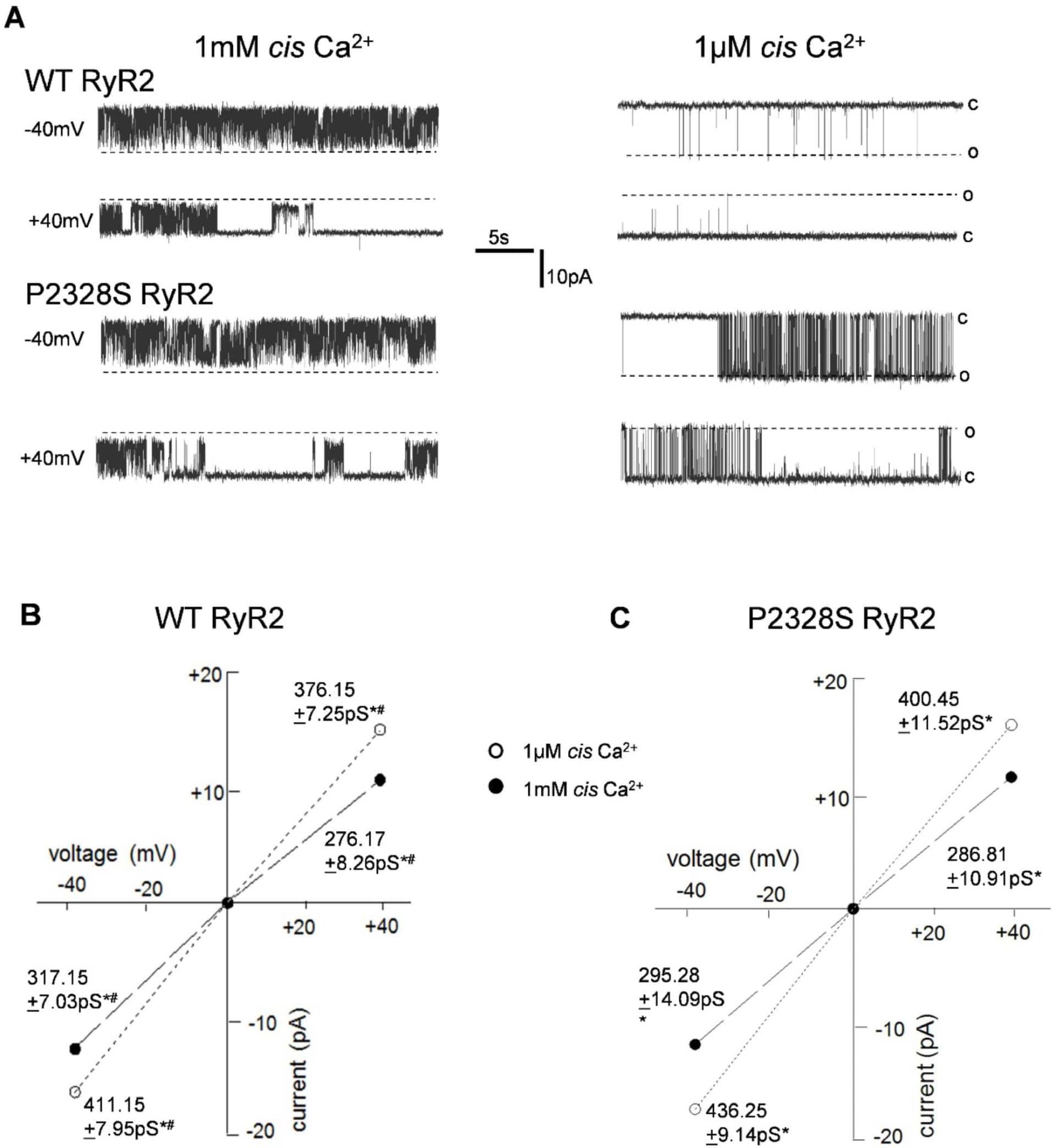
The cardiac ryanodine receptor (RyR2) P2328S mutation is a cause of both catecholaminergic polymorphic ventricular tachycardia (CPVT) [1], leading to fatal ventricular arrhythmias, and atrial arrhythmias including atrial fibrillation. In vivo, RyR2 can exist in various phosphorylation states and in association with regulatory proteins such as FKBP12/12.6 thought to affect channel stability [2,3]. Wild-type (WT) and homozygous RyR2-P2328S inbred 129/Sv mice, age matched across 3-7 months were killed by cervical dislocation in accordance with the UK Animals (Scientific Procedures) Act 1986; hearts were rapidly excised and snap frozen in liquid N2. Homotetrameric RyR2-containing sarcoplasmic reticular (SR) vesicle preparations (each 5-7 hearts) were incorporated into lipid bilayers for single channel recordings and used for co-immunoprecipitation (Co-IP) and western blot (WB). Single channel recordings assessed open probability (Po), mean open time, mean closed time and event frequency at a range of cytoplasmic [Ca2+] ([Ca2+]cyto); 0.1 µM – 1 mM. Recordings with multiple channels were only used for I’F (mean current normalised to maximum current – Po equivalent) determination. Anti-RyR2 Co-IPs were performed and samples run on SDS-PAGE, transferred to PVDF and probed with antibodies for RyR2, FKBP12/12.6 as well as the S2808 and S2814 phosphorylated forms of RyR2. Data are means ± SEM compared by student’s t-test (channel data) or ANOVA with Tukey post hoc (Western blots). Concentration dependences of Po were fit to a Hill equation. Activity in WT and P2328S RyR2 channels (Fig 1) was similar at 1 mM [Ca2+]cyto (Po at -40 mV; 0.55±0.08 vs 0.56±0.07, respectively), but P2328S was significantly more active than WT at 1 µM [Ca2+]cyto (0.24±0.08 vs 0.028±0.07, respectively). This was associated with a >10-fold shift in the AC50 for Ca2+-activation from ~3.5 µM Ca2+ in WT RyR2 to ~320 nM in P2328S channels and an unexpected >1000-fold shift in IC50 for inactivation from ~50 mM in WT channels to ≤7μM in P2328S channels, within systolic [Ca2+] levels. Unexpectedly, the shift in Ca2+-activation was not associated with changes in S2808 or S2814 phosphorylation, or FKBP12/12.6 bound to the channels. These data demonstrate that in vivo derived P2328S-RyR2 channels are more sensitive to cytoplasmic Ca2+ concentrations within the lower diastolic range (0.1-1 µM). This was in the absence of adrenergic challenge and without altered FKBP12 binding or RyR2 S2808/S2814 hyperphosphorylation. This suggests an increased predisposition to CPVT and an alternative mechanism for enhanced channel activity not dependent on hyperphosphorylation or loss of FKBP12/12.6-mediated stabilisation of RyR2 channels.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, C007
Oral Communications: Increased cytoplasmic Ca2+ sensitivity of cardiac ryanodine receptors from the arrhythmic RyR2-P2328S mouse is independent of adrenergic challenge and FKBP12/12.6 regulation.
S. C. Salvage1,3, E. M. Gallant2, N. A. Beard4, S. Ahmad3, H. Valli3, J. A. Fraser3, C. L. Huang3,1, A. Dulhunty2
1. Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom. 2. Eccles Institute of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra, Australian Capital Territory, Australia. 3. Physiology Department, University of Cambridge, Cambridge, United Kingdom. 4. Centre for Research in Therapeutic Solutions, University of Canberra, Canberra, Australian Capital Territory, Australia.
View other abstracts by:
Activity of WT and P2328S RyR2 at 1 mM and 1 �m cytoplasmic (cis) Ca2+
Where applicable, experiments conform with Society ethical requirements.