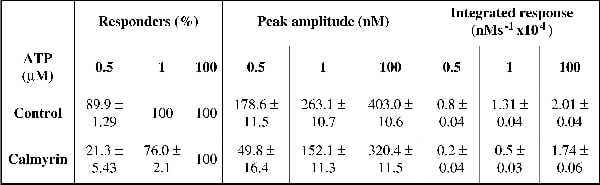
Inositol 1,4,5-trisphosphate receptors (InsP3Rs) are widely-expressed intracellular channels that release Ca2+ from internal stores in response to many physiological stimuli. It is becoming apparent that InsP3Rs form complexes with a multitude of accessory proteins. Many of the proteins that bind to InsP3Rs appear to modulate channel function and Ca2+ release. InsP3Rs are known to be regulated by the ubiquitous EF-hand Ca2+-binding protein calmodulin (CaM). We recently demonstrated that members of the neuronal ‘calcium binding protein’ family (CaBPs), which also bind Ca2+ using EF-hand motifs and have approximately 50% homology with CaM, functionally interact with InsP3 receptors (InsP3R) and inhibit IICR (1). In the present study, we examined the putative interaction of another EF-hand-containing protein, calmyrin, which is ubiquitously expressed and has 56% homology to CaM. We found that calmyrin interacts with the NH2-terminus of InsP3Rs in a Ca2+-independent manner (n=3). Video imaging of fura-2 loaded COS7 cells over-expressing YFP-tagged calmyrin revealed that calmyrin inhibited Ca2+ release from internal stores induced by purinergic agonist (ATP; 0.5, 1 and 100 µM) (refer to table 1 for percentage of responding cells, peak amplitude of the response and integrated response). A mutated protein in which the consensus site for myristoylation was altered (‘calmyrin-G2A’) also inhibited IICR. Unlike wild-type calmyrin, which was largely bound to cellular membranes, the calmyrin-G2A mutant was diffuse. From these data we concluded that calmyrin function is not dependent on its myristoylation and hence membrane targeting. Recombinant calmyrin also inhibited InsP3-dependent 45Ca2+ flux from permeabilised cells (the IC50 of IICR increased from 0.45 ± 0.06 µM to 0.87 ± 0.07 µM in the presence of 10 µM calmyrin (n=3)). The calmyrin-mediated inhibition of Ca2+ release was InsP3R specific since there was no inhibition of caffeine-induced Ca2+ release from ryanodine receptors (peak amplitude of response to 0.5 mM caffeine: 59 ± 8.05 nM (n=10 calmyrin-expressing cells) vs. 67.5 ± 4.69 nM (n=42 control cells); p>0.01). To investigate the mechanism by which calmyrin mediates its effect we performed an InsP3 binding assay using recombinant InsP3R ligand binding domain. In these studies we demonstrated that calmyrin inhibited InsP3 binding to the InsP3R by 22 ± 3% (n=3), in a Ca2+-independent manner. Our data indicate that calmyrin interacts with InsP3Rs and inhibits IICR in a similar manner to CaBPs and CaM.
University College London 2006 (2006) Proc Physiol Soc 3, PC180
Poster Communications: Inhibition of inositol 1,4,5-trisphosphate (InsP3)-induced calcium release (IICR) by the calcium binding protein calmyrin
Anthony Mark Holmes1, Nael Kasri3, Humbert De Smedt3, Jan Parys3, Fraser McDonald2, Martin Bootman1, H Llewelyn Roderick4
1. Calcium Group, Babraham Institute, Cambridge, United Kingdom. 2. Department of Orthodontics and Paediatric Dentistry, UMDS, London, United Kingdom. 3. Laboratorium voor Fysiologie, K. U. Leuven, Leuven, Belgium. 4. Department of Pharmacology, University of Cambridge, Cambridge, United Kingdom.
View other abstracts by:
Table 1. Inhibition of IICR in COS7 cells stimulated with ATP
Where applicable, experiments conform with Society ethical requirements.