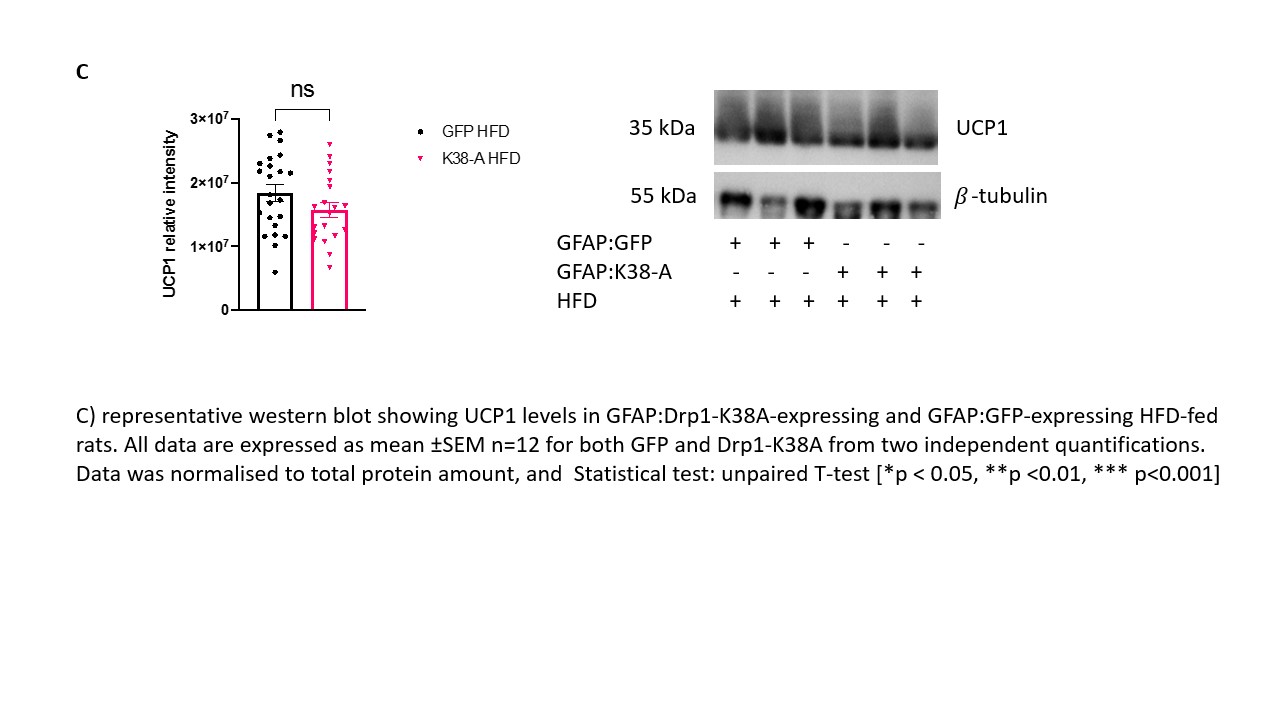
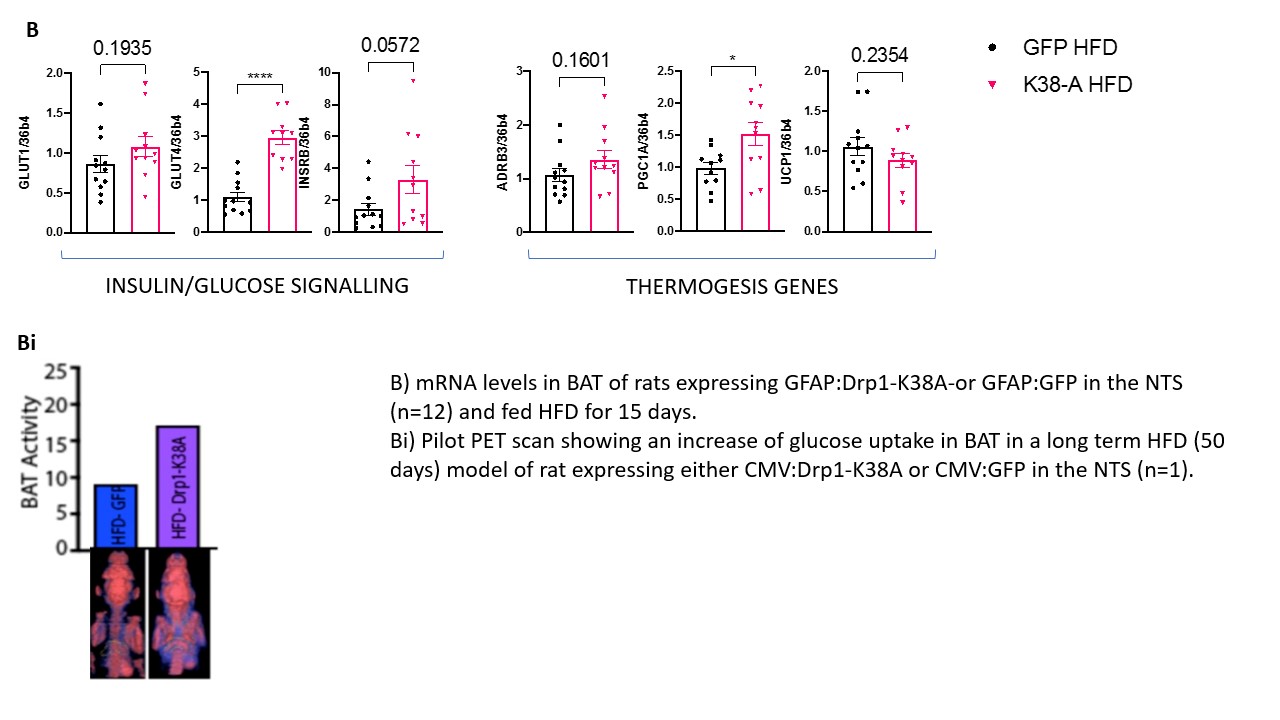
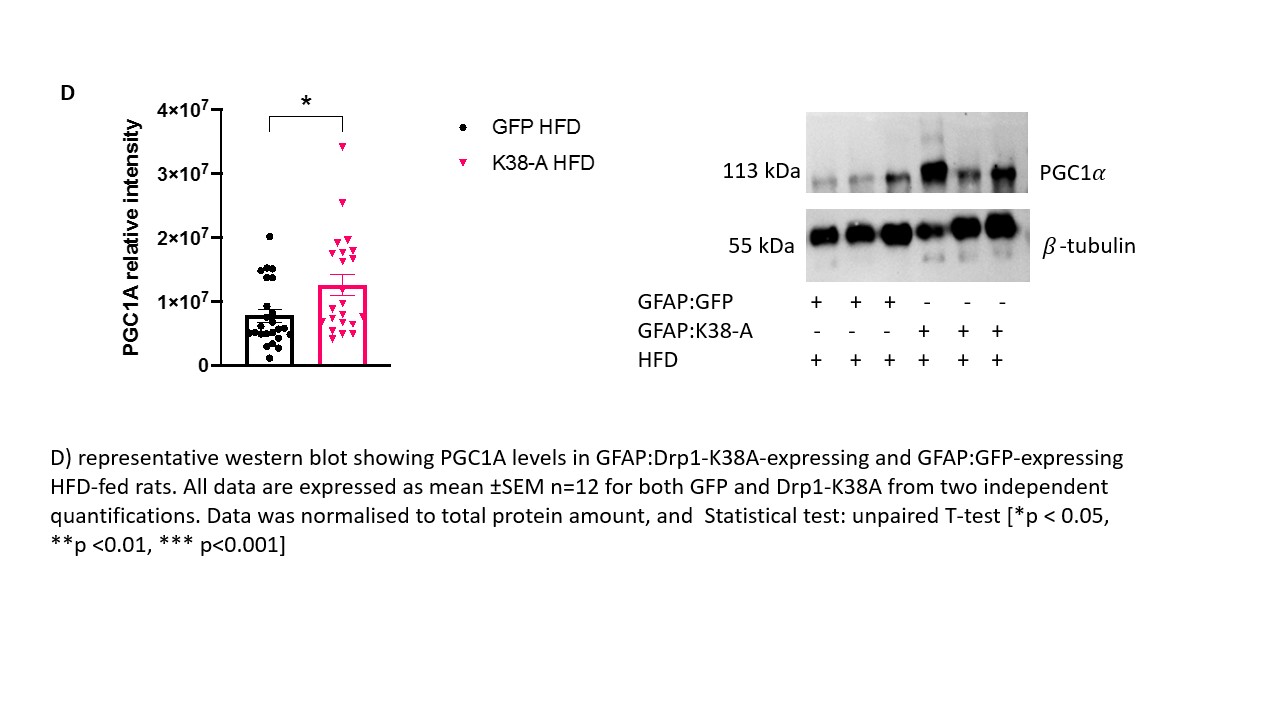
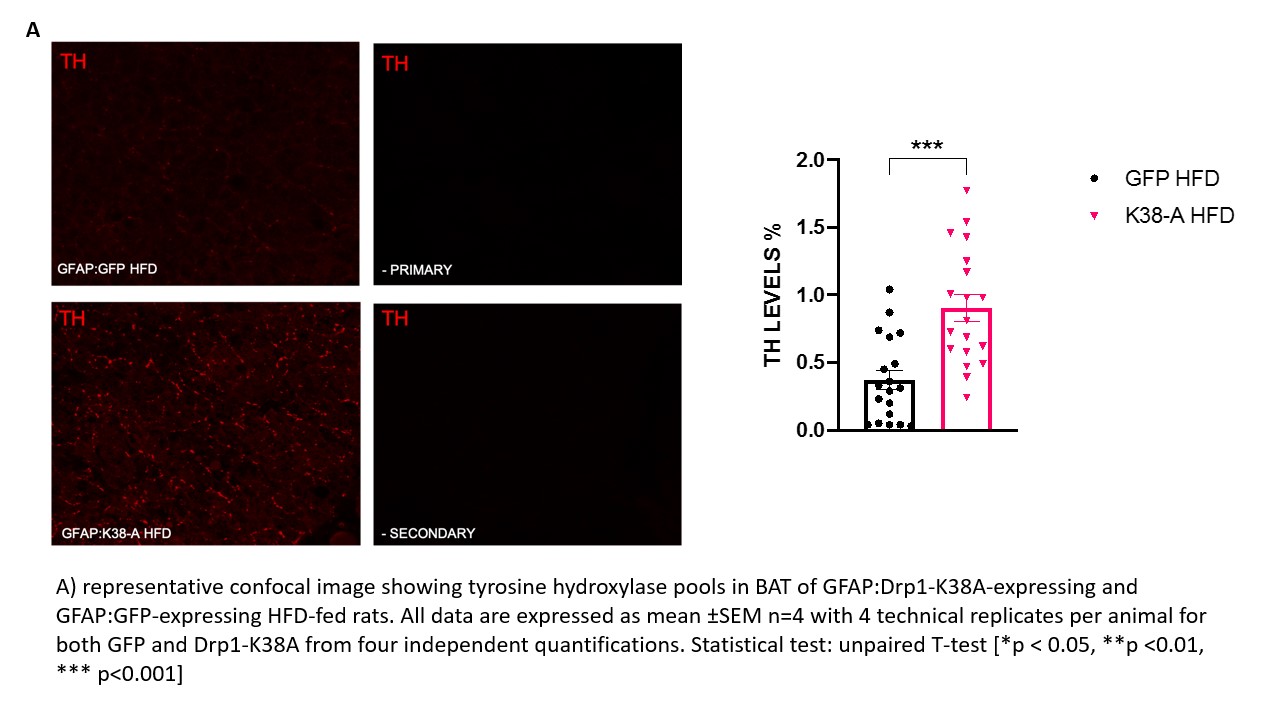
Background and aim: The prevalence of obesity worldwide is increasing sharply, resulting in growing costs for healthcare systems worldwide. The brown adipose tissue (BAT) is being probed as a potential target to treat obesity. BAT activation is driven by the central nervous system (CNS) and circulating glucose and fatty acids. The nucleus of the tractus solitarius (NTS) in the brainstem is an important centre that receives information regarding the nutritional status from the visceral vagal afferents and relays them to the forebrain. Astrocytes are the most abundant cells in the brain that work as energy sensor and provide the right environment for the neurones to respond to altered metabolic status. Their role in regulating systemic metabolism is of growing interest, and evidence shows their involvement in BAT thermogenesis (1). Previous studies have shown that targeting all cells or specifically astrocytes with a dominant-negative form of Drp1 (Drp1-K38A) to inhibit mitochondria fragmentation in rats NTS decreased food intake, restored insulin sensitivity in the NTS and prevented weight gain in high-fat diet (HFD)- fed-obese rats (2). Our main aim is to investigate whether targeting NTS with Drp1-K38A can affect the metabolic profile of BAT and increase adrenergic discharge to the organ and glucose uptake, which associate with enhanced metabolic demand. Materials and methods: The experimental protocol was approved by the ethical committee for the use of animals in research of the Faculty of Biological Sciences,University of Leeds and by the Home Office. 1) Rats (male, n=24) were subjected to stereotactic surgery on day 0 and received an injection of either GFAP:Drp1-K38A or GFAP:GFP in the NTS on day 1 and fed HFD diet for 15 days. On day 16 animals were sacrificed by pentobarbital overdose (60 mg/kg) and BAT dissected. 2) Rats (male, n=3) were fed with HFD for 28 days, submitted to stereotactic surgery on day 29 and injected with an adenovirus expressing CMV:Drp1K138A or CMV:GFP in the NTS on day 30. Rats where then maintained on HFD for additional 15 days and submitted to PET scan. Results: Noradrenaline precursor Tyrosine Hydroxylase (TH) levels in BAT of HFD-fed animals that received GFAP:Drp1K38-A in the NTS are increased 144% when compared to GFAP:GFP controls (p<0.001). The mRNA levels of insulin-dependent glucose transporter 4 (GLUT4) and thermogenic gene peroxisome proliferator-activated receptor gamma (PGC1A) are significantly upregulated in animals that received GFAP:Drp1K38-A in the NTS when compared to controls (p<0.0001 and p<0.05) respectively. On a protein level PGC1A is upregulated (p<0.05). Our Pilot study revealed a potential increase in glucose uptake in BAT of rat fed long-term HFD (50 days) that received CMV:Drp1K38-A when compared to control. Conclusions: Our results suggest that inhibition of mitochondria fission in NTS astrocytes restored TH reservoirs in BAT-essential for beta adrenergic-driven thermogenesis, and regulator of GLUT4 mRNA in BAT(3). Consistently with the PET scan pilot, these results may suggest that glucose uptake in BAT is favoured in animals that received either CMV:Drp1K38-A or GFAP:Drp1K38-A when compared to controls; more studies are required to test this hypothesis.
Future Physiology 2021 (Virutal) (2021) Proc Physiol Soc 47, OC06
Oral Communications: Inhibition of mitochondria fission in astrocytes in the Dorsal Vagal Complex of the brain to target the metabolic profile of brown adipose tissue in high fat diet-fed rats
Arianna Fozzato1, Susan Deuchars1, Beatrice Filippi1
1 University of Leeds, Leeds, United Kingdom
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.